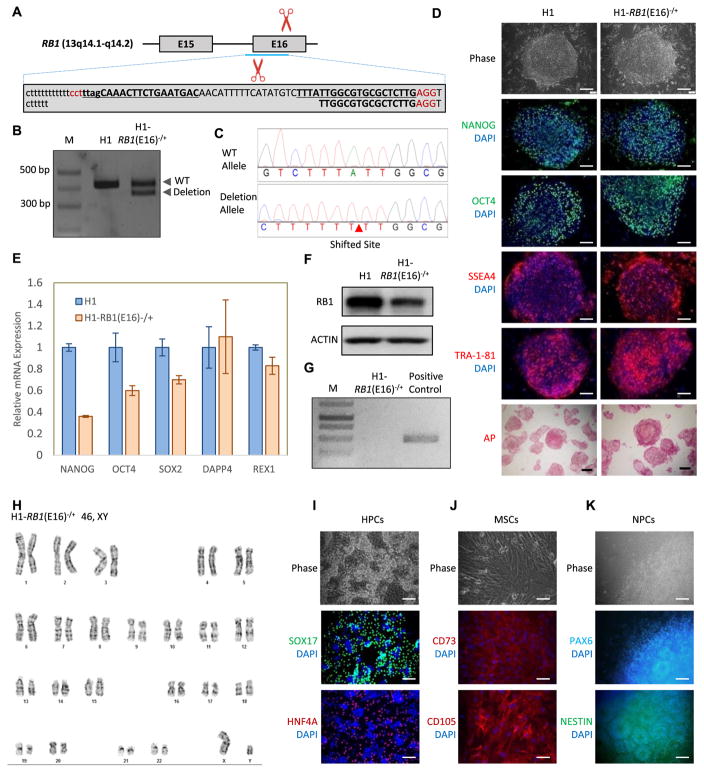
Fig. 1.
Generation and characterization of the RB1 heterozygous knockout hESC line H1-RB1(E16)−/+. (A) Schematic overview of the gene targeting strategy to knockout RB1 exon 16 using CRISPR/Cas9 nickase. RB1 introns and exons are shown in lowercase and uppercase, respectively. The sgRNA target sites are underlined. (B) PCR confirms the deletion of the RB1 exon 16 in the H1-RB1(E16)−/+ line. (C) Sanger sequencing reveals the heterozygous deletion of the RB1 exon16. (D) The H1-RB1(E16)−/+ line expresses hESC pluripotency factors (NANOG and OCT4) and hESC surface markers (SSEA4 and TRA-1-81), and exhibits positive AP activity. Scale bar = 100 μm. (E) qRT-PCR reveals the expression of endogenous human NANOG, SOX2, OCT4, DPPA4, and REX1in H1-RB1(E16)−/+ line. PCR reactions are normalized to GAPDH and plotted relative to expression levels in human H1 ESCs. Error bars indicate ± SEM of triplicates. (F) The H1-RB1(E16)−/+ line presents the lower expression of RB1 protein. (G) The H1-RB1(E16)−/+ line is mycoplasma-free. (H) The H1-RB1(E16)−/+ line shows normal karyotype. (I–K) The H1-RB1(E16)−/+ line maintains pluripotency. Immunofluorescence staining reveals that H1-RB1(E16)−/+ line is capable of differentiating to endodermal (HPCs), mesodermal (MSCs) and ectodermal (NPCs) lineages. Scar bar = 100 μm.