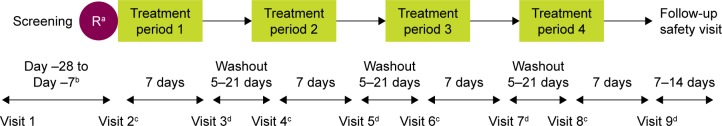
Figure 1.
Study design.
Notes: aAt Visit 2, study site personnel randomized patients in a 1:1:1:1 ratio with an interactive web-based response system into one of the four pre-defined treatment sequences using a four-treatment, four-sequence Williams design.18 The patient, study site personnel, and the study sponsor were blinded to the treatment sequence assigned to a patient. bPatients underwent a washout period of at least 7 days, but not >28 days’ duration prior to returning to the clinic for Visit 2. cDay 1 of each treatment period: in-clinic protocol-defined assessments up to and including the 2-hour post-dose time point. On Day 1 of each treatment period, patients were required to withhold from using short-acting bronchodilators for ≥6 hours prior to administration of the first dose of study drug. dDay 8 of each treatment period: in-clinic protocol-defined assessments up to and including the 2-hour post-dose time point.
Abbreviation: R, randomization.