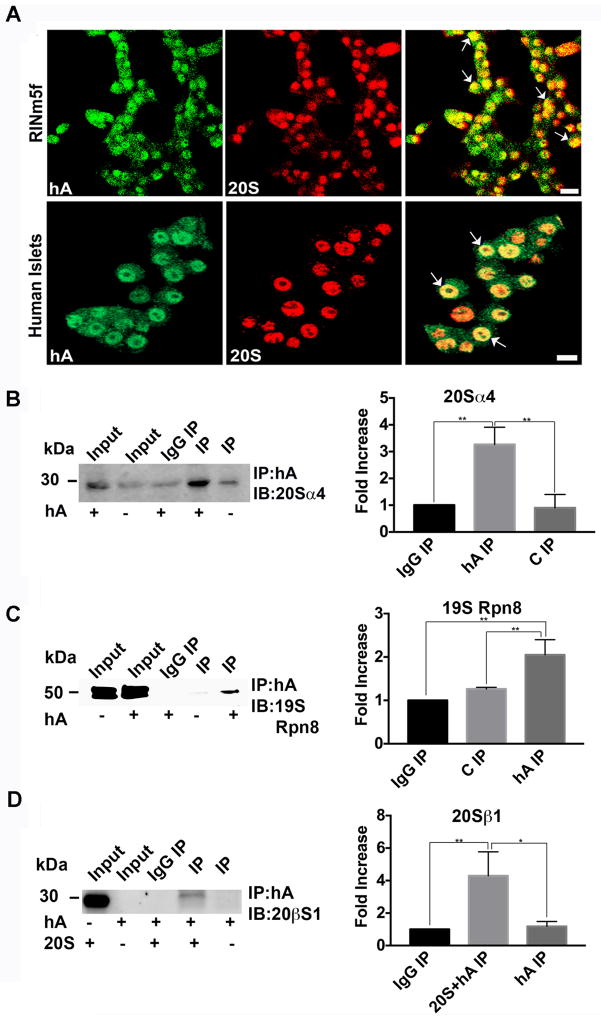
Figure 10. hA interacts with the catalytic and regulatory components of the 26S proteasome complex.
hA was incubated with in RIN-m5F cells and human islets for 24 hours and its interaction with 20s proteasome was assessed by confocal microscopy and immunoprecipitation. (A) hA colocalizes (arrows) with 20S proteasome in the nucleus of RIN-m5F cells (top panel) and human islets (bottom panel) as confirmed by indirect immunocytochemistry. Bars,10 μm. (B–C) hA interacts with the catalytic and lid components of 26S proteasome complex in RINm5F cells. hA was pulled down using hA specific antibody from the nuclear fraction (B) or whole cell extract (C) of hA -treated RIN-m5F cells and immunoblotted with antibodies against 20Sα4 subunit (B) and 19SRpn8 (C) subunits of the 26S proteasome complex. (D) hA interacts with 20Sβ1 in vitro to form a heterocomplex. Synthetic hA and purified 20S complex were co-incubated and immunoprecipitated using anti- hA antibody as bait. Anti-20Sβ1 antibody was used to confirm pull down of hA/20S immunocomplex. Significance was established at * p<0.05,** p<0.01 n=3, ANOVA followed by Tukey’s post hoc comparison test (B–D, histograms).