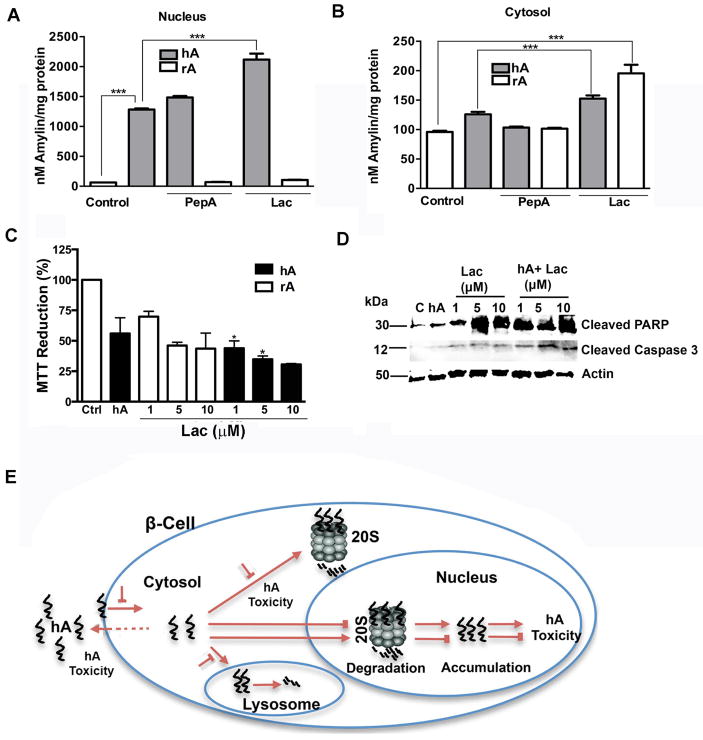
Figure 11. Inhibition of proteasome proteolytic function accelerates nuclear accumulation and toxicity of hA in pancreatic cells.
(A–B) hA was incubated with cells in the presence or absence of lactacystin for 24 hours. The extent of hA accumulation in the presence and absence of lactacystin (1uM) or pepstatin A (1μM) in (A) nucleus and (B) cytosol is revealed by ELISA. Significance was established at * p<0.05,** p<0.01 and *** p<0.001, n=3–6, ANOVA followed by Tukey’s post hoc comparison test. (C–D) Analysis of hA cytotoxicity in response to proteasomal inhibition. Dose dependent effect of lactacystin (1–10μM) on hA toxicity was analyzed by (C) MTT stress assay and (D) PARP/Caspase 3 cleavage assay. Significance was established at * p<0.05, n=3–6, ANOVA followed by Tukey’s post hoc test, hA vs Lac treatments. (E) A schematic representation of endocytotic-regulated hA internalization followed by proteasome-mediated degradation and detoxicification of hA in pancreatic cells is depicted. Conversely, inhibition of hA internalization and proteasome functions can lead to excessive accumulation of hA on the plasma membrane and intracellularly leading to its aggregation and toxicity.