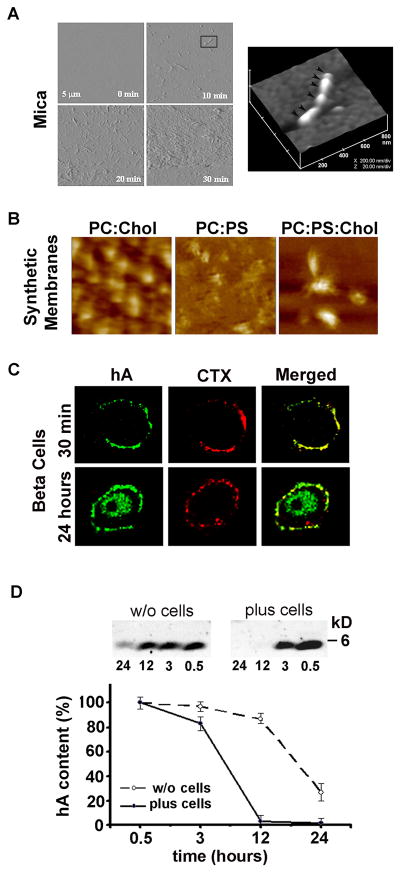
Figure 2. High resolution microscopic analysis of hA aggregation on solid surfaces and membranes.
(A) Tapping mode time-lapse AFM was used to capture structural intermediates, oligomers and fibrils, during hA aggregation on mica. Note a time-dependent structural transition of hA from small fibrils (early stage of hA aggregation, 10 min) to amyloid-like dense deposits (late stage of hA aggregation, 20–30 min). All micrographs on the left panel are 5×5 μm. 3-D AFM image of a single full-grown fibril on mica (inset, 10 min) reveals linear alignment of several hA oligomers and their bi-directional extension into a fibril (depicted by arrowheads). Micrograph is 800 × 800 nm scale. (B) AFM analysis of membrane-directed hA self-assembly. High-resolution 2D AFM analysis revealed distinct deposition pattern and morphology of hA aggregates on synthetic lipid membranes. Note the clustering of hA aggregates on cholesterol-containing membranes, PC:Chol (3.2:0.8 mol:mol) and PC:PS:Chol (2.3:1:0.8 mol:mol:mol). In contrast, hA aggregates were less compact and homogenously distributed across cholesterol-free membranes-PC:PS (2.8:1.2 mol:mol). In contrast to mica (Figure 2A), no fibrils were detected on either membranes (Figure 2B). Micrographs are 2×2 μm. (C) Confocal microscopy analysis of binding and clustering of hA on the β-cell PM. Rinm5F cells were exposed to hA (20 μM) for 30 min or 24h. Cells were then washed and fixed prior to immunochemical analysis. hA specific antibody (green) was used to analyze peptide’s accumulation on the plasma membrane and intracellularly. Fluorescently-labeled lipid-raft marker cholera toxin (CTX, red) was added to cell during last 30 min. of hA incubations to localize lipid rafts micro domains on the cell plasma membrane. Note hA and CTX co-clustering on the cell plasma membrane (yellow puncta) and time-dependent hA internalization in a single β-cell indicating hA extracellular clearance. (D) Clearance of extracellular hA by pancreatic β-cell revealed by western blot. hA (20 μM) was added to Rinm5F cells or cell-free buffer and the changes in hA content in solution were analyzed over 24 by western blot approach. hA (4 kDa) was detected using amylin-specific antibody. Note the accelerated clearance of hA from solution containing cells. The slow decrease in hA content in cell-free solution is due to delayed hA aggregation and precipitation from solution. Due to its toxicity, peptide solvent HFIP was omitted from these studies.