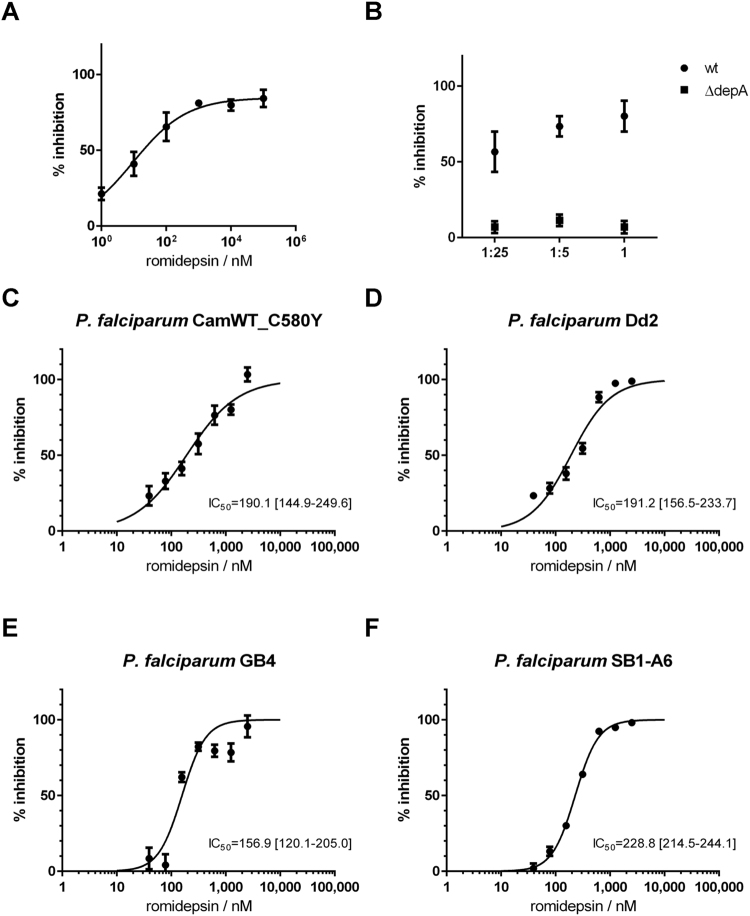
Figure 5.
Romidepsin surpasses common drug resistance mechanisms and interferes with P. falciparum HDAC activity. Inhibition of HDAC activity of P. falciparum NF54 nuclear protein extracts in the presence of (A) increasing concentrations of romidepsin or (B) n-butanol extracts of approximately 5 mL (1×), 1 mL (1:5) and 200 µL (1:25) of culture supernatants (LB, 72 h, 30 °C) of C. sp. 968 wildtype (wt) and depA-null mutant (∆depA) as measured by endpoint immunodetection of remaining acetylated histone substrate following incubation. Results are normalized to vehicle control of 1.3% DMSO (0% inhibition) and absence of P. falciparum nuclear protein extract (100% inhibition). Dose-response curve of romidepsin against P. falciparum CamWT_C580Y (C, resistant to artemisinin), Dd2 (D, resistant to chloroquine, pyrimethamine and mefloquine), GB4 (E, resistant to chloroquine) and SB1-A6 (F, resistant to cytochrome bc1 inhibitors such as atovaquone). 0% inhibition is adjusted for parasite growth in vehicle control (1% DMSO) and 100% inhibition is matched to that of 250 nM chloroquine. IC50 is indicated together with the 95% confidence interval returned after fitting a curve to the normalized data using the least squares method with variable slope.