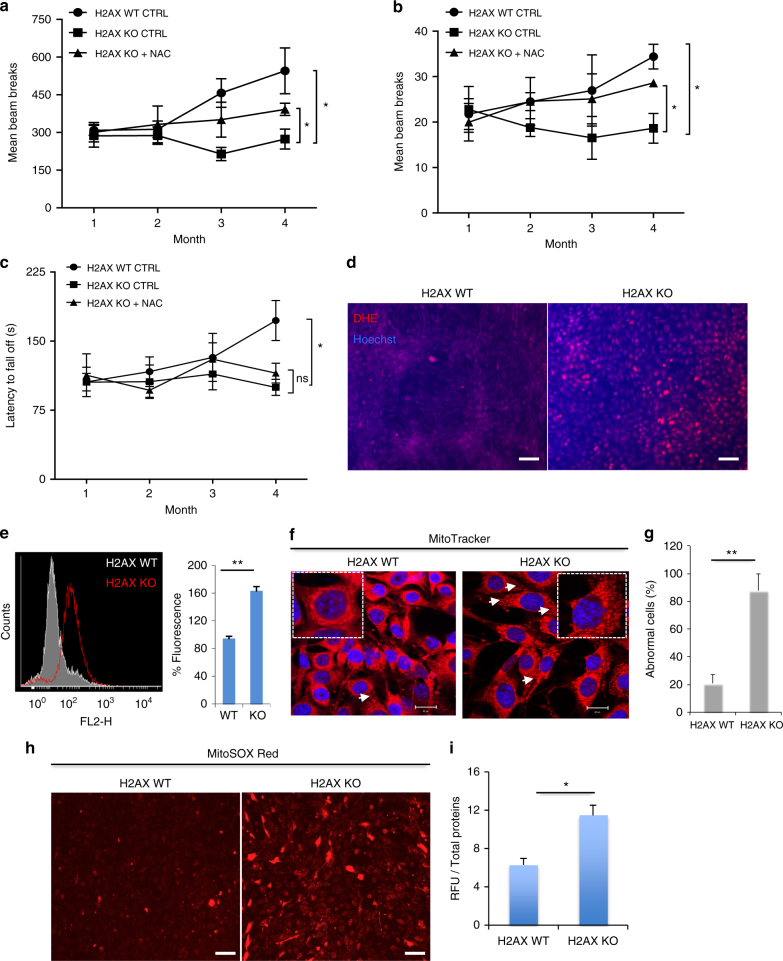
Fig. 2.
Oxidative stress is involved in H2AX loss-induced neurobehavioral deficits. a, b N-acetyl-cysteine (NAC) treatment prevents disturbed general motor activity. Open-field testing demonstrated that N-acetyl-cysteine (NAC 20 mM) significantly reduces impaired motor activity in H2AX knockout mice as measured by horizontal activity (a) or vertical activity (b), y-axis, number of beam breaks, x-axis, month. Data indicate the average of locomotor activity over 45 min each month. n = 5 (means ± s.e.m.) for H2AX wild type control group, n = 7 (means ± s.e.m.) for H2AX knockout control group, and n = 8 (means ± s.e.m.) for H2AX knockout treated with NAC. *P < 0.05. c Rotarod tests were also performed on mice used in a, b. Testing was conducted each month from weaning to 4 months, where each mouse was given three rounds of training, and latency to fall off the rotarod was measured at it accelerated from 4 to 40 rpm over 5 min; y-axis, the latency to fall off in seconds, x-axis, month. n = 5 (means ± s.e.m.) for H2AX wild type control group, n = 7 (means ± s.e.m.) for H2AX knockout control group, and n = 8 (means ± s.e.m.) for H2AX knockout treated with NAC. *P < 0.05, ns non-significant (P = 0.1482). d, e H2AX deletion leads to accrued ROS levels. Control cells (H2AX WT), as well as mutant mouse embryonic fibroblasts (H2AX KO) were incubated with dihydroethidium (DHE) and used for ROS detection by microscopy. Cells were counterstained using Hoechst (d). The raw DHE fluorescence was analyzed by flow cytometry (e), (Left, raw DHE fluorescence; right, quantification of the fluorescence mean). Data are means ± s.d.; n = 3. **P < 0.001. f, g Mitochondrial morphology was analyzed in both wild-type and H2AX mutants. Cells were stained with 500 nM MitoTracker and visualized on a Zeiss LSM 700 confocal laser scanning microscope; f representative image. When stained with the mitochondrial marker Mitotracker, the mitochondria in the H2AX KO cells appeared swollen and less filamentous than those in the control cells. The percentage of cells with these abnormalities is estimated in g and data are means ± s.d.; n = 3. **P < 0.001. h Representative images obtained by fluorescence microscopy of MitoSOX Red staining in control and H2AX mutant cells. Fluorescence intensity was quantified using Plate Reader (i), and data are means ± s.d.; n = 3, *P < 0.05. Scale bars: d and h, 100 μm. For all data, statistical significance was determined by a two-tailed, unpaired Student’s t-test