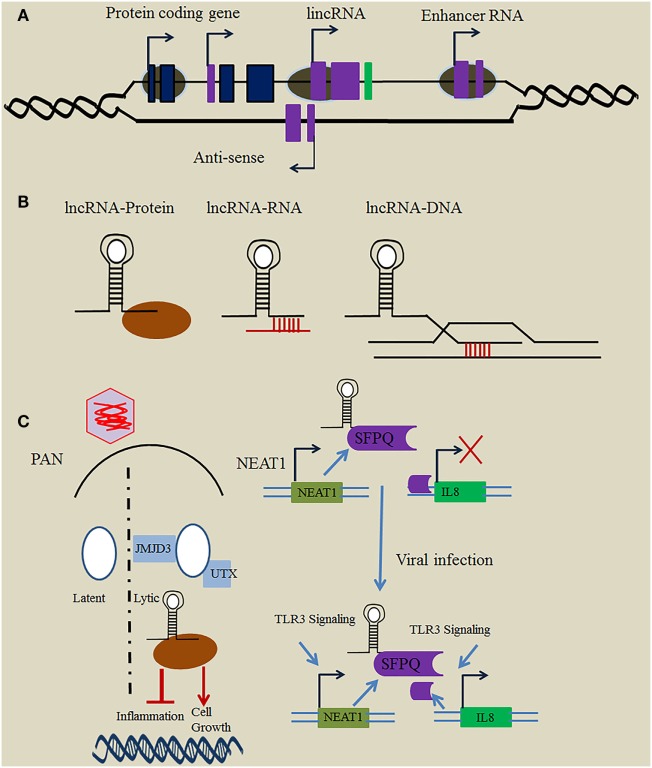
Figure 1.
Mechanism employed by long non-coding RNA (lncRNA) in immune regulation. (A) Loci of immune-related lncRNAs relative to protein-coding genes. In general, immune-related lncRNAs are transcribed by RNAP II and classified relative to the position of the neighboring protein-coding gene into different types, including intronic lncRNA, long intergenic ncRNA (lincRNA), enhancer RNA (eRNA), and antisense lncRNA. (B) Mechanisms employed by lncRNAs. lncRNAs use a variety of basic modules to perform their regulatory functions in the cytosol and nucleus through lncRNA–protein, lncRNA–RNA, and lncRNA–DNA interactions. (C) Function of herpesviruses’ lncRNAs in immune regulation. The Kaposi’s sarcoma-associated herpesvirus (KSHV)-encoded lncRNA polyadenylated nuclear (PAN) binds the histone-modifying complex (demethylases JMJD3 and UTX) which plays an important role in the switch from latent to lytic infection. PAN also subverts the host immune response and modulates viral gene expression through binding with PRC2 to promote cell growth and survival, and to repress the inflammatory response. Nuclear paraspeckle assembly transcript 1 (NEAT1) binds several proteins such as SFPQ, and plays a critical role in regulation of the innate immune response mechanism through the transcriptional regulation of numerous antiviral genes upon herpesvirus infection.