Abstract
Solitary fibrous tumors are primary mesenchymal tumors, which may occur in any part of the body. Overall, these tumors are considered to have intermediate malignant potential with 5- and 10-year metastasis-free and overall disease-specific survival rates of 74% and 55%, and 89% and 73%, respectively (Demicco et al, 2012). Herein we present an unusual case of solitary fibrous tumors involving the ischioanal fossa in a 19-year-old woman with radiologic-pathologic correlation. This case was complicated by extensive tumor vascularity and was thus managed with preoperative embolization followed by en bloc surgical resection.
Keywords: Solitary fibrous tumor, Embolization, Ischioanal, Hemangiopericytoma
Introduction
Solitary fibrous tumors (SFTs) are primary mesenchymal spindle cell neoplasms, which usually originate from the pleura, but which, however, can uncommonly arise throughout the body. SFTs most commonly occur in the fifth and sixth decades of life [1]. Herein we present an unusual case of SFT of the ischioanal fossa involving a 19-year-old woman.
Case report
A 19-year-old woman initially presented to an outside hospital with right lower quadrant pain and leukocytosis. Computed tomography (CT) of the abdomen and pelvis was performed that revealed a 7 × 6-cm lobulated heterogeneously enhancing mass within the right ischioanal fossa or perirectal fat with mild mass effect and associated leftward deviation of the rectum (Fig. 1). The patient was subsequently referred to the colorectal surgery clinic at our own institution. On consultation, she complained of right lower quadrant-pelvic pain (+7/10), which was pulsatile, sharp, without radiation, and constantly present; she did not report any mitigating or exacerbating factors. She also reported 1 episode of bright red blood per rectum. No pain related to bowel movements. She does menstruate monthly, with most recent menstruation events not being particularly heavy or long. She has a hormonal intrauterine device placed 3 years prior to presentation.
Fig. 1.
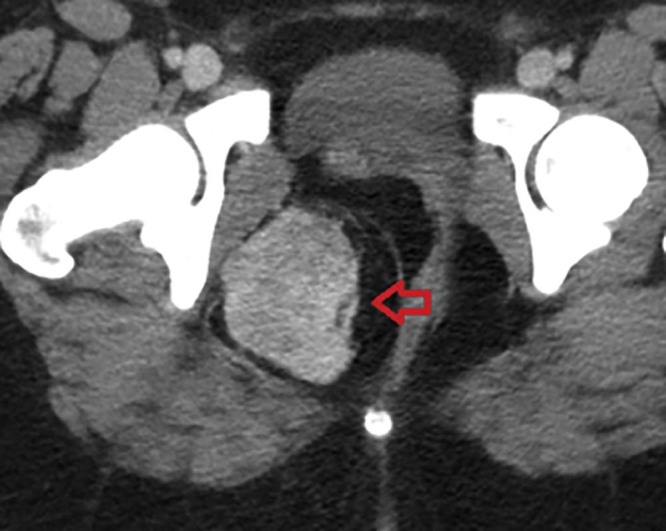
Computed tomography scan showing the enhancing mass in the right ischioanal fossa (red arrow).
Medical history was significant for a pituitary tumor, treated medically, and history of polycystic ovarian syndrome, for which she was not currently receiving treatment. Review of systems was unremarkable. Physical examination was remarkable for a smooth, soft mass along the right wall of the rectum palpated on digital rectal examination. Otherwise, the remainder of the physical examination was unremarkable. Specifically, perineal inspection showed normal skin, without evidence of a lesion or mass, and anoscopy demonstrated no mucosal abnormality.
Magnetic resonance imaging (MRI) was obtained for further characterization of the mass, which, consistent with the previous CT findings, demonstrated a large lobulated perineal soft tissue mass occupying the right ischioanal fossa with intense postcontrast enhancement (Fig. 2, Fig. 3). There was mass effect on the vagina and the rectum (Fig. 4). Based on these findings, a differential diagnosis of angiomyxoma vs schwannoma was suggested. Subsequently, CT-guided biopsy of the mass was performed, which was complicated by moderate intralesional hemorrhage. This was addressed with gel-foam embolization of the needle tract and responded well to conservative further management.
Fig. 2.
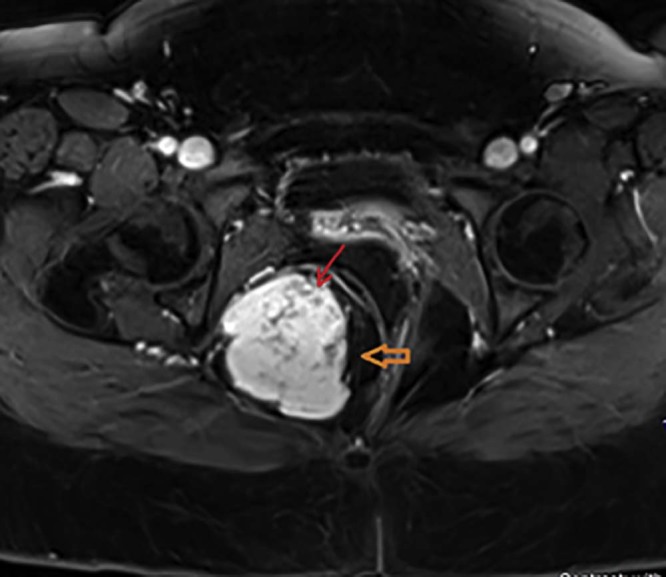
Postcontrast T1-weighted magnetic resonance imaging axial image demonstrating intense contrast enhancement (orange arrow) of the right ischioanal fossa mass. The nonenhancing serpentine structures (red arrow) represent intratumoral vascularity.
Fig. 3.
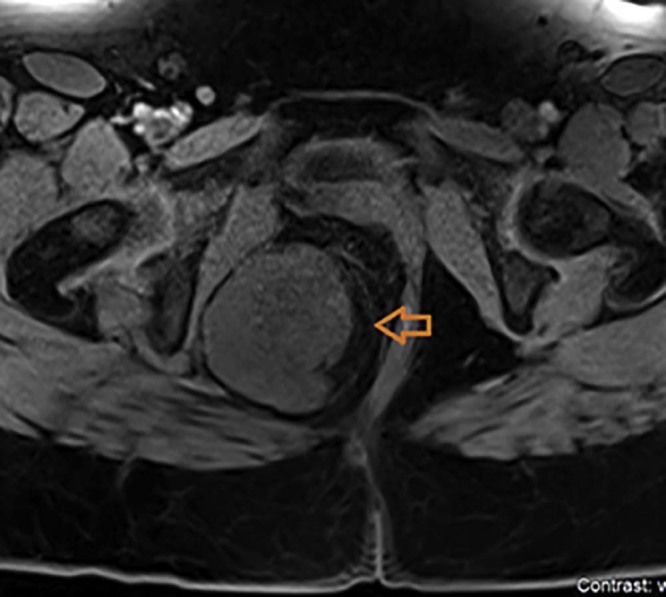
Noncontrast T1-weighted magnetic resonance imaging axial image of the pelvis demonstrating an isointense mass (orange arrow) in the right ischioanal fossa.
Fig. 4.
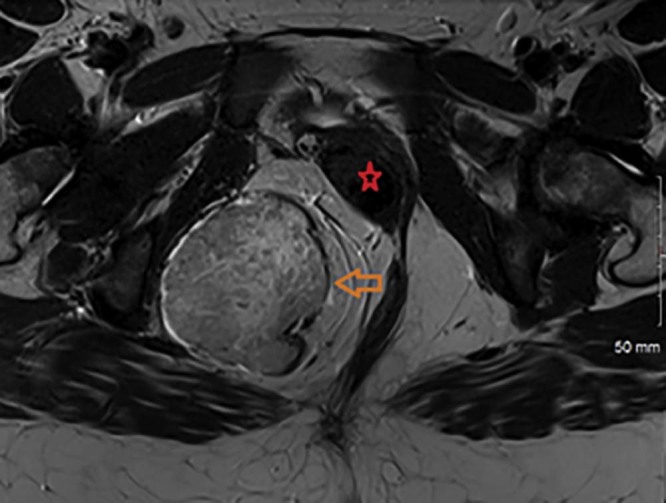
T2-weighted magnetic resonance imaging axial image of the pelvis showing a large heterogeneous mass (orange arrow) occupying the right ischioanal fossa and demonstrating areas of hyper- and hypo-signal intensity. Mass effect on the vagina and the rectum noted (red star).
The sections were stained by the immunohistochemical technique for leukocyte common antigen, cytokeratin, and CD34, CD31, and S100 protein. The neoplastic cells were strongly reactive for CD34 but were essentially nonreactive with the other aforementioned antibodies. Based on histopathology and immunohistochemical studies, a diagnosis of hemangiopericytoma or SFT was made.
The case was presented at our local tumor board, and decision was made to proceed with neoadjuvant embolization followed by surgical resection of the mass with particular concern for the vascularity identified on MRI and evidenced by the intralesional hemorrhage during percutaneous biopsy. Neoadjuvant embolization was also recommended to attain preoperative tumor shrinkage and thus hopefully achieve better surgical resection margins.
The preoperative embolization was carried out a week before the planned surgery with hopes to allow enough time for tumor shrinkage while still limiting neoangiogenesis. The procedure was performed via a left common femoral approach using a 10-cm 6F Pinnacle sheath (Terumo Corporation, Somerset, NJ). Pelvic angiogram was performed with a 5F Soft-Vu Omniflush catheter (Angiodynamics, Latham, NY)(Fig. 5). The right internal iliac artery was catheterized using a Kumpe catheter (Cook Medical, Canton, IL) and a Glidewire (Terumo Corporation). Right internal iliac angiogram confirmed the tumor blush and identified the main arterial supply from the right internal pudendal artery. This was selectively catheterized using a Progreat microcatheter (Terumo Corporation) and embolization was performed with 300-500 µ polyvinyl alcohol particles (Cook Medical) (Fig. 6, Fig. 7, Fig. 8). Approximately 2 vials of polyvinyl alcohol were used to completely devacularize the tumor. The access was closed with the Mynx plug (Cardinal Health, Dublin, OH). The patient was admitted overnight for observation and was uneventfully discharged the following day in stable condition.
Fig. 5.
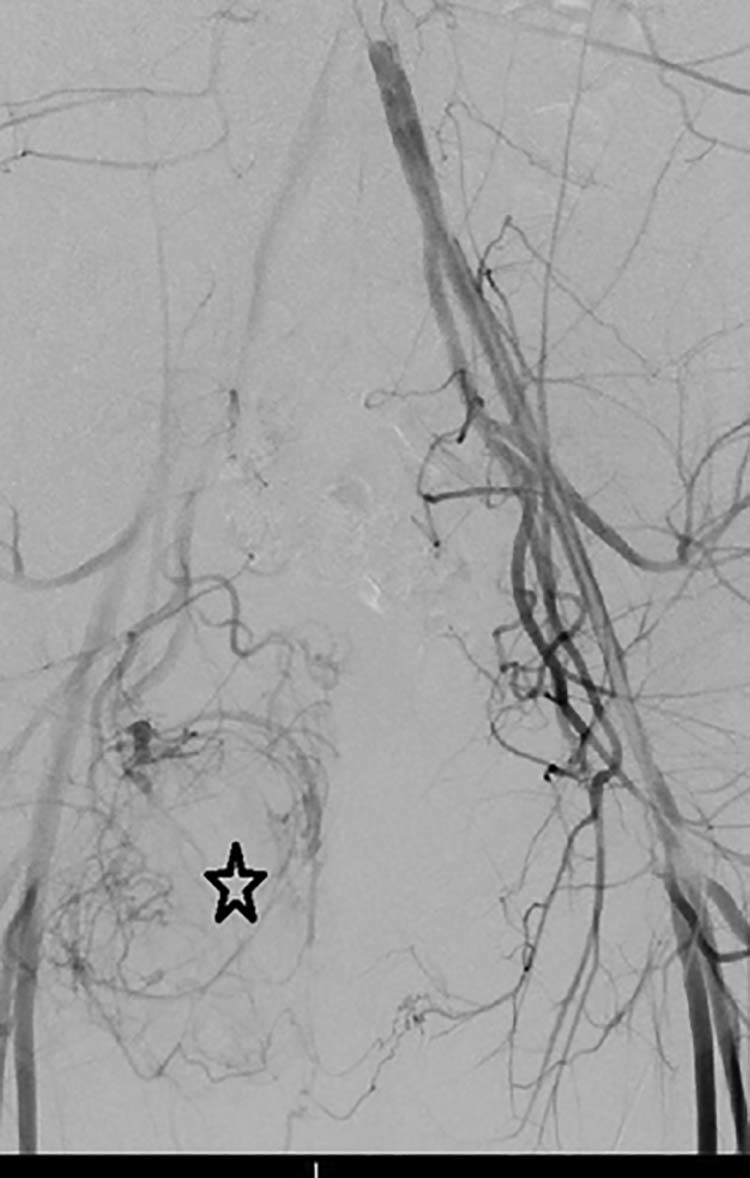
Pelvic angiogram demonstrating tumor vascularity (black star) in the right hemipelvis.
Fig. 6.
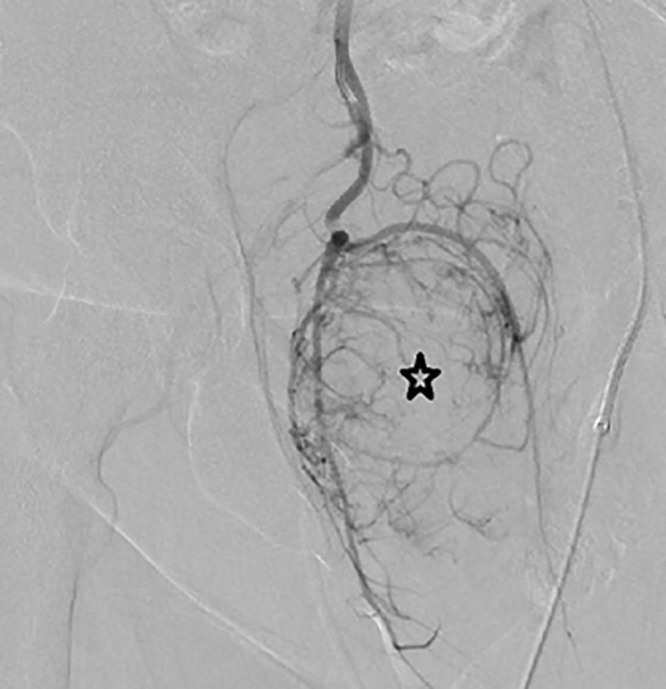
Selective catheterization of the right internal pudendal artery with angiogram confirming tumor vascularity (black star).
Fig. 7.
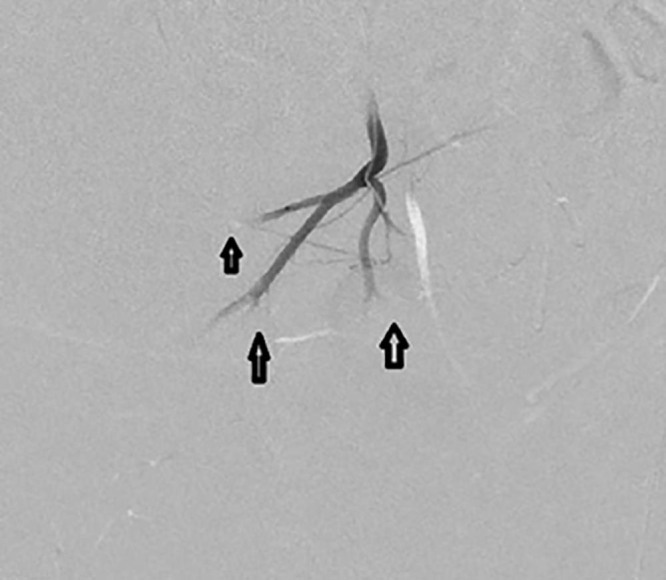
Postembolization angiogram demonstrating pruning of the feeding vessels and stasis (black arrows).
Fig. 8.
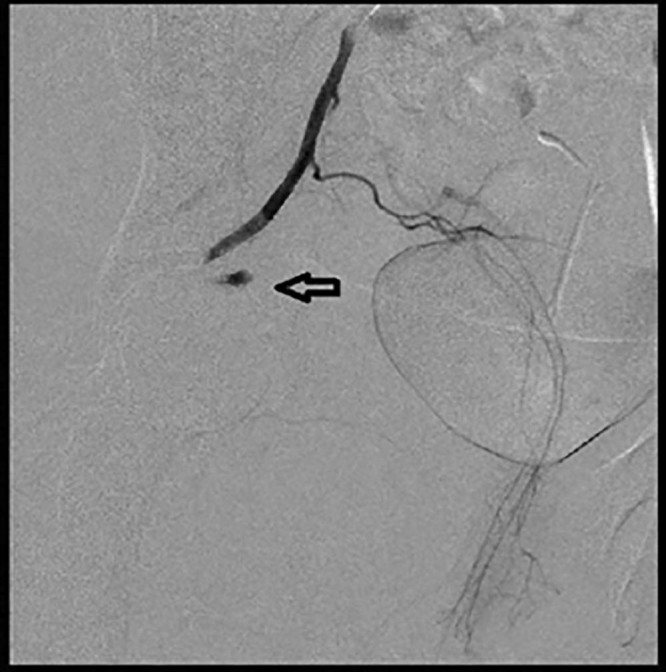
Postembolization angiogram demonstrating pruning of the feeding vessels and stasis (black arrow).
Operative details
The patient was taken to the operating theater 1 week after embolization. General anesthesia was established. The patient was placed in prone jack-knife position. Standard preparation and drape were performed. A vertical incision was made on top of the right ischioanal fossa, 2 cm away from the anal verge. The incision was deepened, the tumor was palpated, and sharp dissection around the tumor with bovie and harmonic scalpel was performed, with care taken to avoid cutting into the tumor itself and maintaining a margin of normal fatty tissue. The rectum was not involved and easily separated from tumor. At the lateral deep margin, the tumor appeared to be densely adherent to the periosteum, which was sharply incised en bloc with tumor. At the cephalad, deep margin part of the levator ani muscle was removed along with tumor mass. The tumor was thus successfully resected en bloc and delivered directly to our pathology department. The procedure was performed with minimal blood loss of approximately 50 cc. Copious irrigation was performed. A drain was placed inside the wound cavity and pulled out from a separate stab incision.
Pathology findings
Gross pathology demonstrated a 12.0 × 6.5 × 4.0-cm mass surrounded by an asymmetric rim of adipose tissue. The mass was well circumscribed but not encapsulated and had a fleshy tan to pink cut surface (Fig. 9).
Fig. 9.
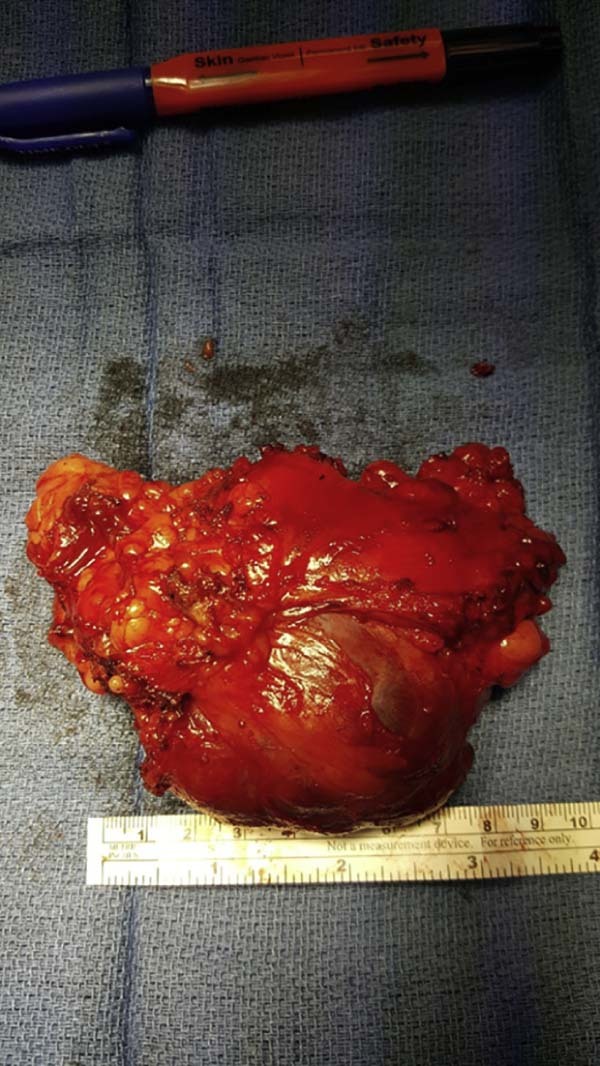
Gross pathology of en bloc resected mass.
Microscopic analysis revealed a well-circumscribed spindle cell proliferation composed predominately of hypercellular areas separated by thick bands of hyalinized cartilage. The hypercellular areas were characterized by branching thin-walled vessels with a staghorn-like appearance (Fig. 10, Fig. 11). Higher power examination revealed the cellular areas to be composed of oval to spindle-shaped nuclei containing a dispersed chromatin pattern and small nucleoli. Scattered areas of myxoid change, stromal edema, and fibrosis were also seen (Fig. 12). Mitotic figures were infrequent with approximately 1 mitotic figure per 10 high-power fields.
Fig. 10.
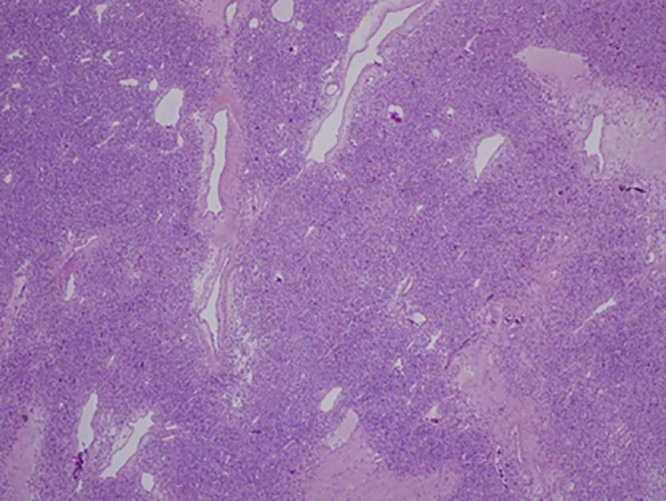
Low power view of a cellular area with prominent thin-walled vasculature and zones of dense fibrosis (H&E, ×40)
Fig. 11.
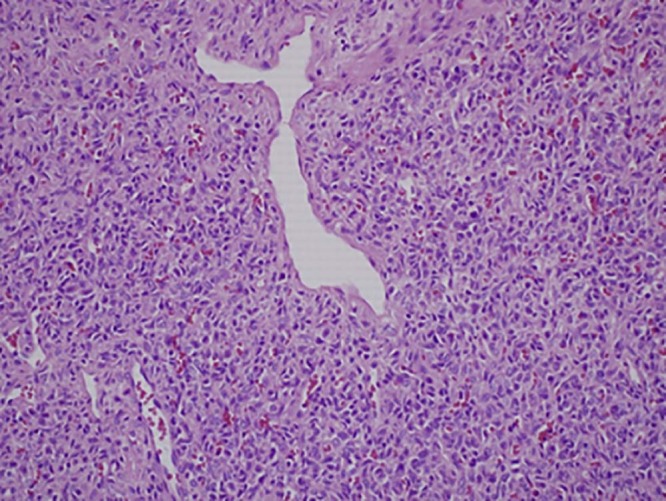
High-power view of a cellular area demonstrating staghorn-like vessels. (H&E, ×200)
Fig. 12.
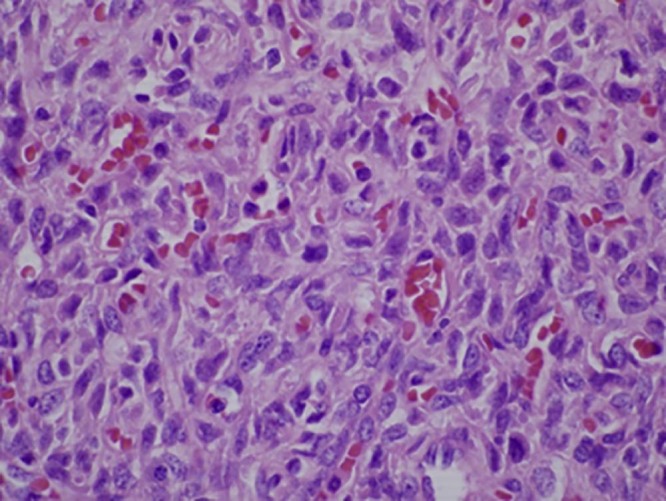
High-power view demonstrating oval to spindle-shaped nuclei with a dispersed chromatin pattern (H + E, ×600)
Immunohistochemistry was performed revealing the neoplastic cells to express STAT6 (Fig. 13) and CD34 reactivity without reactivity for S100 protein, common leukocyte antigen, cytokeratin, or CD31. A final diagnosis of extrapleural SFT was given.
Fig. 13.
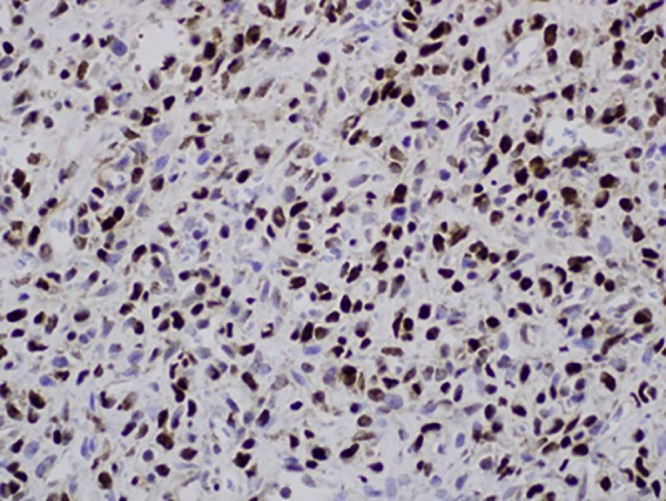
Immunohistochemical stain for STAT6 demonstrating strong nuclear staining.
Discussion
SFTs are rare soft tissue neoplasms, most commonly arising in the pleura although several case reports have described extrathoracic locations [2], [3]. It was first reported in 1931 [4] and has since acquired several synonyms including benign mesothelioma, subpleural fibroma fibromyxoma, and pleural fibroma [5].
Clinical presentation is often secondary to bulk-related symptoms or nondescript pain in the affected region. Other interesting features of this tumor are the various possible paraneoplastic symptoms such as hypoglycemia secondary to secretion of insulin-like peptides [6]. The rarity of these neoplasms warrants full imaging, pathologic review, and discussion at multidisciplinary tumor board.
With only 5 reported cases of anorectal SFT, literature on management of these tumors is scant at best [7], [8], [9], [10], [11]. Two of these cases involve SFTs in the ischioanal fossa, 1 of rectal origin which reoccurred in the perineum, and the other originating in the mesorectum and involved both the rectum and uterus. Average tumor size was 10 cm (range 7-13 cm). All cases were treated surgically. Two patients had excision of the tumor through an abdominal approach, with sparing of the rectum [8], [9]; 2 patients underwent an abdominal perineal resection [7], [11], and 1 underwent a perineal extraperitoneal procedure.
Follow-up data were available in only 3 of the 5 cases, and ranged from 6 months to 13 years. Both patients who underwent abdominal perineal resection experienced local recurrence at 6 months and at 13 years, respectively [7], [11]. One of the patients received neoadjuvant radiation to the mass and neoadjuvant percutaneous embolization of bilateral internal pudendal arteries with the aim to reduce the tumor size prior to re-excision. Although excision with clear margins should always be the goal in treating anorectal SFT, optimal margin size remains unknown. Radiotherapy should be reserved for complicated cases such as SFTs with malignant features, positive margins postresection, or unresectability [12].
Extrapleural SFTs generally present as well-circumscribed, often partially encapsulated masses with a mean size between 5 and 7 cm. Cross section will usually reveal a multinodular appearance with a whitish color and firm consistency. Areas of myxoid and hemorrhagic degeneration, as seen in this case, are considered uncommon [3], [13]. Microscopic examination reveals the so-called patternless architecture with a combination of hypo- and hypercellular areas separated by thick hyalinized bands of collagen. Some examples will be dominated by a hemangiopericytoma-like pattern of small branching vessels with a staghorn pattern [14], [15], [16]. The individual tumor cells are ovoid to spindle-shaped and have scant to modest amounts of pale cytoplasm with indistinct borders. The nuclei have a dispersed chromatin with a vesicular pattern. Mitotic activity is generally low, rarely exceeding 3 mitoses per 10 high-powered fields. Higher mitotic indices indicate the likelihood of a malignant phenotype [7], [17]. Immunohistochemically, SFTs are characteristically reactive with antibodies directed against CD34 and STAT6. Less commonly, they are positive for epithelial membrane antigen and smooth muscle antibody [16].
The pathologic diagnosis of SFT, in contradistinction to other mesenchymal tumors, can be difficult [18]. Bcl-2 and CD99 immunohistochemistry may be used to distinguish a hemangiopericytoma from an SFT because an SFT is usually positive for Bcl-2 and CD99 [17], [19]. Hemangiopericytoma immunohistochemical analysis is usually positive for CD34 and vimentin, and negative for Bcl-2, CD99, c-kit, factor VIII, desmin, alpha-smooth muscle actin, S100 protein, epithelial membrane antigen, and keratin.
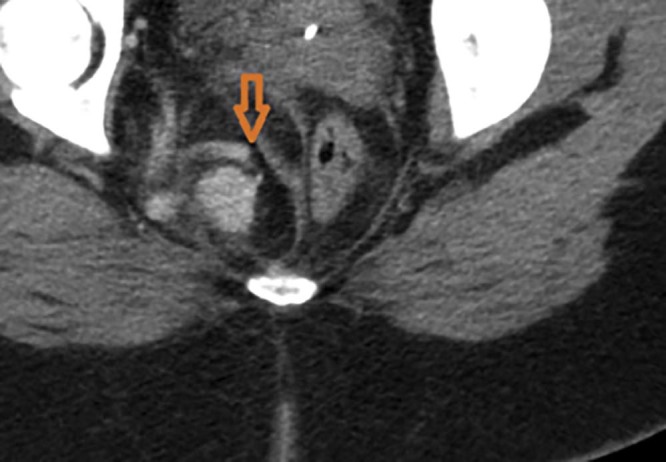
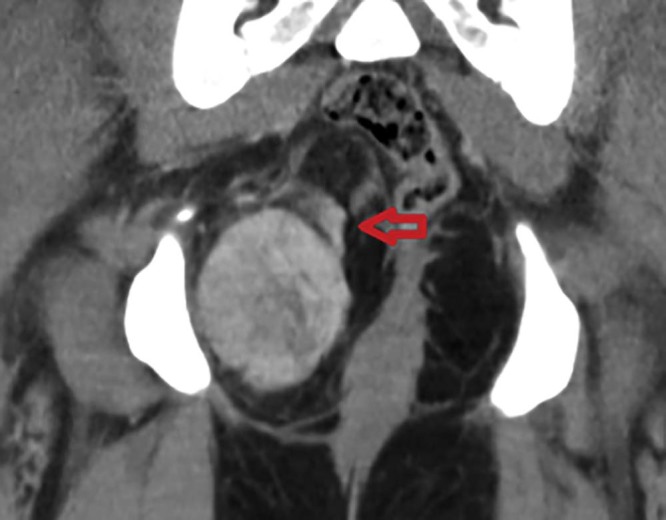
MRI is usually chosen as the method for detecting the organ of origin of a pelvic mass. On MRI, SFT shows intermediate signal intensity on T1-weighted images and heterogeneous signal on the fat-saturated T2-weighted images. The hypointense areas on the T2 images correlating with fibrous or sclerotic tissue [20], [21] and the T2 hyperintense areas correspond to the more cellular, myxoid areas or cystic changes [22], [23], [24]. Following contrast administration enhancement pattern depends on the histologic factors like cellularity and the amount of fibrous or myxoid tissue. Up to 25% of the tumors may show intratumoral vessels with high flow, as was seen in our case [25]. Wignall et al reported about 35% (12 of 34 cases) of the tumors in their series had collateral vessels in the peritumoral tissues. This feature may help to narrow the differential; however, in their series, this did not follow a particular histologic subtype [1]. Similar imaging appearance was seen in our case on the CT (see Fig. 14, Fig. 15). Differentials to consider would be hemangiopericytoma, leiomyosarcoma, liposarcoma, neurogenic tumors such as schwannoma, and malignant fibrous histiocytoma (MFH). Neurogenic tumors often occur in lateral walls of the pelvis with moderate or marked enhancement. MFH and leiomyosarcoma generally have nonspecific imaging findings, which do not facilitate a definitive diagnosis; however, a low-signal-intensity intratumoral septum on T2-weighted images may indicate a diagnosis of MFH [26].
Fig. 14.
Axial contrast-enhanced computed tomography showing a large peritumoral vessel feeding the mass (orange arrow).
Fig. 15.
Coronal reformation of the contrast-enhanced computed tomography showing the peritumoral vessel (red arrow).
Because the tumor in our case was very vascular, as appreciated by the moderate to severe intralesional hemorrhage during the CT-guided biopsy, we opted for neoadjuvant embolization of the tumor prior to surgical resection. The intraoperative blood loss of just 50 cc suggests that preoperative embolization may very well have been effective in the management of this patient.
The clinical behavior of SFTs is variable. Although most are benign and slow growing with a favorable prognosis, SFT can behave aggressively and even result in mortality [7], [27]. Higher rates of local recurrence are reported for extrapleural SFTs possibly due to smaller excision margins in relation to the anatomic localization of the tumor. Positive margins, tumors size greater than 10 cm, and malignant histology are all risk factors of local recurrence of extrapleural SFTs [13], [28], [29].
Data thus suggest complete resection is critical in the management of SFT. Hypervascular SFTs may increase the technical difficulty and perioperative risk of surgery; and hence possibly impact the chances of successful en bloc resection with negative margins of these tumors. Thus, we believe that neoadjuvant angiography and embolization should be considered in the management of SFTs although additional research is indicated to establish safety and efficacy of this approach.
References
- 1.Wignall O.J., Moskovic E.C., Thway K., Thomas J.M. Solitary fibrous tumor of the soft tissues: review of the imaging and clinical features with histopathologic correlation. AJR Am J Roentgenol. 2010;195(1):W55–W62. doi: 10.2214/AJR.09.3379. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher C.D.M., Bridge J.A., Lee J.C. Extrapleural solitary fibrous tumour. In: Fletcher C.D.M., Bridge J.A., Hogendoorn P.C.W., Mertens F., editors. WHO classification of tumours of soft tissue and bone. 4th ed. IARC Press; Lyon, France: 2013. pp. 80–82. [Google Scholar]
- 3.Suster S., Nascimento A.G., Miettinen M., Sickel J.Z., Moran C.A. Solitary fibrous tumors of soft tissue: a clinicopathologic and immunohistochemical study of 12 cases. Am J Surg Pathol. 1995;19(11):1257–1266. doi: 10.1097/00000478-199511000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Klemperer P., Rabin C.B. Primary neoplasms of the pleura. Arch Pathol. 1931;11:385–412. [Google Scholar]
- 5.Hasegawa T., Hirose T., Seki K., Yang P., Sano T. Solitary fibrous tumor of the soft tissues. An immunohistochemical and ultrastructural study. Am J Clin Pathol. 1996;106:325–331. doi: 10.1093/ajcp/106.3.325. [DOI] [PubMed] [Google Scholar]
- 6.Cotterill A.M., Holly J.M., Davies S.C., Coulson V.J., Price P.A., Wass J.A. The insulin-like growth factors and their binding proteins in a case of non-islet-cell tumor-associated hypoglycaemia. J Endocrinol. 1991;131:303–311. doi: 10.1677/joe.0.1310303. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa T., Matsuno Y., Shimoda T., Hasegawa F., Sano T., Hirohashi S. Extrathoracic solitary fibrous tumors: their histological variability and potentially aggressive behavior. Hum Pathol. 1999;30:1464–1473. doi: 10.1016/s0046-8177(99)90169-7. [DOI] [PubMed] [Google Scholar]
- 8.Dudkiewicz M., Deschenes J.L., Bloom C., Gordon P.H. Solitary fibrous tumor of the ischioanal fossa: report of a case and review of the literature. Dis Colon Rectum. 2004;47:535–537. doi: 10.1007/s10350-003-0075-9. [DOI] [PubMed] [Google Scholar]
- 9.Joe B.N., Bolaris M., Horvai A., Yeh B.M., Coakley F.V., Meng M.V. Solitary fibrous tumor of the male pelvis: findings at CT with histopathologic correlation. Clin Imaging. 2008;32:403–406. doi: 10.1016/j.clinimag.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 10.Mourra N., Lewin M., Sautet A., Parc R., Flejou J.F. Epithelioid solitary fibrous tumor in the ischioanal fossa. Virchows Arch. 2005;446:674–676. doi: 10.1007/s00428-005-1255-x. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida R., Takada H., Iwamoto S., Uedono Y., Kawanishi H., Yoshioka K. A solitary fibrous tumor in the perianal region with a 13-year follow-up: report of a case. Surg Today. 1999;29:642–645. doi: 10.1007/BF02482992. [DOI] [PubMed] [Google Scholar]
- 12.Robinson L.A. Solitary fibrous tumor of the pleura. Cancer Control. 2006;13:264–269. doi: 10.1177/107327480601300403. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa T., Matsuno Y., Shimoda T., Hasegawa F., Sano T., Hirohashi S. Extrathoracic solitary fibrous tumors: their histological variability and potentially aggressive behavior. Hum Pathol. 1999;30(12):1464–1473. doi: 10.1016/s0046-8177(99)90169-7. [DOI] [PubMed] [Google Scholar]
- 14.Chan J.K. Solitary fibrous tumour—everywhere, and a diagnosis in vogue. Histopathology. 1997;31:568–576. doi: 10.1046/j.1365-2559.1997.2400897.x. [DOI] [PubMed] [Google Scholar]
- 15.Mentzel T., Bainbridge T.C., Katenkamp D. Solitary fibrous tumour: clinicopathological, immunohistochemical, and ultrastructural analysis of 12 cases arising in soft tissues, nasal cavity and nasopharynx, urinary bladder and prostate. Virchows Arch. 1997;430:445–453. doi: 10.1007/s004280050054. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen G.P., O'Connell J.X., Dickersin G.R., Rosenberg A.E. Solitary fibrous tumor of soft tissue: a report of 15 cases, including 5 malignant examples with light microscopic, immunohistochemical, and ultrastructural data. Mod Pathol. 1997;10:1028–1037. [PubMed] [Google Scholar]
- 17.Fukunaga M., Naganuma H., Ushigome S., Endo Y., Ishikawa E. Malignant solitary fibrous tumor of the peritoneum. Histopathology. 1996;28:463–466. doi: 10.1046/j.1365-2559.1996.297345.x. [DOI] [PubMed] [Google Scholar]
- 18.Enzinger F.M., Smith B.H. Hemangiopericytoma: an analysis of 106 cases. Hum Pathol. 1976;7:61–82. doi: 10.1016/s0046-8177(76)80006-8. [DOI] [PubMed] [Google Scholar]
- 19.Cristi E., Perrone G., Battista C., Benedetti-Panici P., Rabitti C. A rare case of solitary fibrous tumor of the presacral space: morphological and immunohistochemical features. In Vivo. 2005;19:777–780. [PubMed] [Google Scholar]
- 20.Jeong A.K., Lee H.K., Kim S.Y., Cho K.J. Solitary fibrous tumor of the parapharyngeal space: MR imaging findings. AJNR Am J Neuroradiol. 2002;23:473–475. [PMC free article] [PubMed] [Google Scholar]
- 21.Kim H.J., Lee H.K., Seo J.J., Shin J.H., Jeong A.K., Lee J.H. MR imaging of solitary fibrous tumors in the head and neck. Korean J Radiol. 2005;6:136–142. doi: 10.3348/kjr.2005.6.3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chun H.J., Byun J.Y., Jung S.E., Kim K.H., Shinn K.S. Benign solitary fibrous tumour of the pre-sacral space: MRI findings. Br J Radiol. 1998;7 doi: 10.1259/bjr.71.846.9849394. [DOI] [PubMed] [Google Scholar]
- 23.Kakihara D., Yoshimitsu K., Eto M., Matsuura S., Honda H. MRI of retroperitoneal solitary fibrous tumor in the suprarenal region. AJR Am J Roentgenol. 2007;188:W512–W514. doi: 10.2214/AJR.05.0537. [DOI] [PubMed] [Google Scholar]
- 24.Casas J.D., Balliu E., Sa′nchez M.C., Mariscal A. Benign solitary fibrous tumor of the retroperitoneum: radiological features. CMIG Extra Cases. 2004;28:50–53. [Google Scholar]
- 25.Cardinale L., Allasia M., Ardissone F., Borasio P., Familiari U., Lausi P. CT features of solitary fibrous tumour of the pleura: experience in 26 patients. Radiol Med. 2006;111:640–650. doi: 10.1007/s11547-006-0062-z. [DOI] [PubMed] [Google Scholar]
- 26.Agaimy A., Gaumann A., Schroeder J., Dietmaier W., Hartmann A., Hofstaedter F. Primary and metastatic high-grade pleomorphic sarcoma/malignant fibrous histiocytoma of the gastrointestinal tract: an approach to the differential diagnosis in a series of five cases with emphasis on myofibroblastic differentiation. Virchows Arch. 2007;451:949–957. doi: 10.1007/s00428-007-0495-3. [DOI] [PubMed] [Google Scholar]
- 27.Demicco E.G., Park M.S., Araujo D.M., Fox P.S., Bassett R.L., Pollock R.E. Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol. 2012;25(9):1298–1306. doi: 10.1038/modpathol.2012.83. [DOI] [PubMed] [Google Scholar]
- 28.Gold J.S., Antonescu C.R., Hajdu C., Ferrone C.R., Hussain M., Lewis J.J. Clinicopathologic correlates of solitary fibrous tumors. Cancer. 2002;94:1057–1068. [PubMed] [Google Scholar]
- 29.Fukunaga M., Naganuma H., Nikaido T., Harada T., Ushigome S. Extrapleural solitary fibrous tumor: a report of seven cases. Mod Pathol. 1997;10(5):443–450. [PubMed] [Google Scholar]


