Abstract
Aims: The number of ovarian follicles and ovulations after human chorionic gonadotrophin (hCG) was given at various doses during pregnancy were investigated in rats.
Methods: On designated days of pregnancy, rats were injected with hCG at 13.00 hours and the number of ova were observed. The same rats were then killed at 13.00 hours the next day. The ovaries were cut into complete sections. All sections were observed and the size of each follicle was measured.
Results: The number of large (≥550 µm), healthy, non‐atretic follicles (Graafian follicles) ranged from 1.5 to 11 during pregnancy. Ovulation was induced in rats at any stage of pregnancy with intramuscular injections of 40 or 50 IU hCG, but 10 IU had no effect and 20 or 30 IU had an incomplete effect. The number of ovulations after 40 IU hCG was 2–12 and there was not a significant difference between the number of ovulations and the number of large, healthy follicles at any point in the pregnancy.
Conclusion: Graafian follicles existed through the pregnancy. Results suggest that although hCG induces ovulation in large ovarian follicles (≥550 µm) during pregnancy, the responsiveness to hCG is lower than in recurrent estrus‐stage rats. (Reprod Med Biol 2004; 3: 201–204)
Keywords: graafian follicle, ovarian follicle, ovulation, pregnancy, rats
INTRODUCTION
ADMINISTRATION OF HUMAN chorionic gonadotropin (hCG) to pregnant guinea pigs, mice or hamsters induces ovulation in all stages of pregnancy, 1 , 2 , 3 but in rats, it has been reported that ovulation is only induced immediately before delivery, 4 either because there are no large ovarian follicles to respond to hCG, or because they are not responsive to hCG even if they are present. Therefore, we investigated the follicular kinetics in pregnant rats and administered hCG in each stage of pregnancy to assess its effect on ovulation.
MATERIALS AND METHODS
Animals
WISTAR‐IMAMICHI RATS from the Institute for Animal Reproduction, Ibaraki, Japan, were maintained at 23 ± 2°C, with illumination from 07.00 to 21.00 hours. The midpoint of the dark period (02.00 hours) was defined as ‘midnight, colony time’ and all times are reported in relation to that. Food and water were supplied ad libitum. A previous study 5 in rats had shown that there are marked changes in the number of follicles with age (days), therefore in the present study a vaginal smear check was initiated at the age of 10 weeks and experiments were commenced at the age of 12 weeks. Vaginal smears were taken daily, before 09.00 hours, for at least two consecutive cycles before any treatment was administered. Only animals showing regular 4‐day cycles were used in the experiments.
Experiment 1: Follicular development in the pregnant rat
Proestrus‐stage rats were caged with males at a ratio of 1:1, and the day of a sperm‐positive smear was designated as day 1 of pregnancy, after which 10 rats were killed at 13.00 hours on each day of pregnancy (Fig. 1). The ovaries were removed, fixed for 24 h, and then dehydrated and embedded in paraffin by routine procedures, followed by complete serial sectioning (10 µm section) and staining with Mayer's hematoxylin‐eosin solution. All sections were observed and the size of each follicle was measured using a micrometer. In follicles with oocytes, the mean of the major and minor axes was regarded as the follicular size.
Figure 1.

The day of a sperm‐positive smear was designated as day 1 of pregnancy and then 10 rats were killed at 13.00 hours each day of pregnancy. Non‐atretic healthy follicles were differentiated from atretic follicles according to the criteria described by Braw and Tsafiriri. 6
We excluded atretic follicles, which were differentiated from non‐atretic healthy follicles (Graafian follicles) according to the criteria described by Braw and Tsafiriri; 6 non‐atretic healthy follicles (i.e. oocytes) were in the resting stage of prophase (dictyate), except immediately before ovulation, and pyknosis of granulosa cells and cell debris in the antrum were absent.
Experiment 2: Effect of human chorionic gonadotrophin dose on ovulation in the pregnant rat
On designated days during pregnancy (Table 1), groups of five rats were injected intramuscularly with 10, 20, 30, 40 or 50 IU hCG (Gonatropin; Teikokuzoki, Tokyo, Japan) at 13.00 hours and then killed 24 h later with sodium pentobarbital and ether. The oviducts were flushed to recover the ova, and hyaluronidase was used to disperse the cumulus cells from the newly ovulated oocytes so that they could be counted with the aid of a binocular dissecting microscope.
Table 1.
Administration of human chorionic gonadotropin during pregnancy
| Day of pregnancy | Human chorionic gonadotrophin treatment (IU) | ||||
|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | |
| Number of ovulations/number of animals | |||||
| 4 | 0/5 (0) | 2/5 (6.2) | 5/5 (6.6) | 5/5 (6.5) | 5/5 (6.1) |
| 8 | 0/5 (0) | 1/5 (4.0) | 3/5 (6.7) | 5/5 (7.0) | 5/5 (6.6) |
| 12 | 0/5 (0) | 1/5 (3.0) | 5/5 (4.8) | 5/5 (5.8) | 5/5 (5.5) |
| 16 | 0/5 (0) | 1/5 (4.0) | 4/5 (5.9) | 5/5 (6.0) | 5/5 (5.8) |
| 20 | 0/5 (0) | 0/5 (0) | 4/5 (6.4) | 5/5 (6.8) | 5/5 (7.2) |
| Total (%) | 0 | 20.0 | 84.0 | 100.0 | 100.0 |
The rats were injected at 13.00 hours and killed 24 h later to recover the number of ova (in parentheses).
Experiment 3: Induction of ovulation in the pregnant rat
On days 1–22 of pregnancy, rats were injected with 40 IU hCG (i.m.) at 13.00 hours and then killed 24 h later to record the number of ova in the oviduct, which was then regarded as the number of ovulations.
RESULTS
Experiment 1: Follicular development in the pregnant rat
ON DAYS 1–22 of pregnancy, the number of non‐atretic healthy follicles, greater than 550 µm in size, remained between 1.5 and 11.1 on average (Fig. 1).
Experiment 2: Effect of human chorionic gonadotrophin dose on ovulation in the pregnant rat
Of the rats injected with 10, 20 or 30 IU hCG, 0, 20 and 84%, respectively, ovulated, whereas in the rats receiving 40 or 50 IU hCG, ovulation occurred in 100% of the animals (Table 1).
Experiment 3: Induction of ovulation in the pregnant rat
All of the rats injected with 40 IU hCG ovulated during pregnancy, and the number of ova shed paralleled the number of non‐atretic large follicles greater than 550 µm (Fig. 2).
Figure 2.
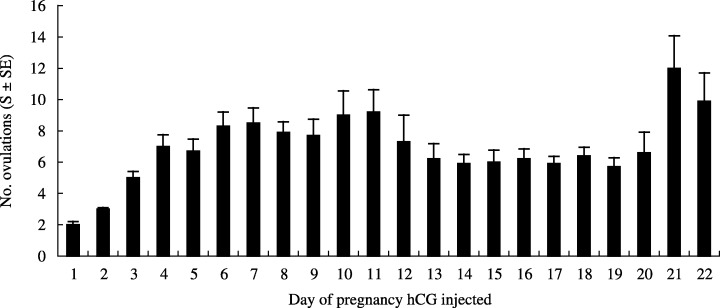
Ten rats were killed each day of pregnancy after 40 IU human chorionic gonadotrophin (hCG) was administered and the number of ova observed in the oviduct was regarded as the number of ovulations.
DISCUSSION
IN THE PRESENT study using Wistar‐Imamichi rats, large ovarian follicles measuring 550 µm or more with a normal morphology, which may respond to hCG and lead to ovulation, were present in all stages of pregnancy. This differs from the finding reported by Greenwald that large ovarian follicles appear on day four or later of pregnancy in Sprague‐Dawley rats. 4 This discrepancy may be related to differences in both the strain and age of rats used in the two studies. It has been confirmed that in Wistar‐Imamichi rats, age markedly influences the number of healthy ovarian follicles measuring less than 550 µm, but not the number greater than that size, so we cannot conclude that the difference was related solely to the age difference.
We conducted Experiment 2 to clarify the relationship between the dose of hCG and ovulation and found that 40 or 50 IU induced 100% ovulation, which suggested that at least 40 IU hCG was required to induce ovulation in pregnant rats, and that dose was confirmed in Experiment 3. Greenwald reported that hCG did not induce ovulation, even if there were a few large ovarian follicles, 4 however 20 IU hCG, which was used in Greenwald's study, may be insufficient to induce ovulation.
In Greenwald's study, rats during recurrent estrus, but not pregnant rats, were induced to ovulate with 10 IU hCG and Greenwald speculated that this was because ovarian follicles in pregnant rats became refractory to hCG. 4 Kagabu et al. reported that the minimum dose of hCG required for ovulation in rats during recurrent estrus was 0.59 IU, also suggesting that large ovarian follicles during pregnancy were refractory to hCG. 7 Singh and Greenwald speculated that the refractoriness of large ovarian follicles to hCG was related to their physiological immaturity. 8
In the present study, 40 IU hCG induced ovulation in all stages of pregnancy and at no point of pregnancy was there any significant difference between the number of ovulations and the number of large healthy ovarian follicles measuring 550 µm. Our results suggest that although hCG induces ovulation in large ovarian follicles (≥550 µm) during pregnancy, the responsiveness to hCG is lower than in recurrent estrus‐stage rats. Whether or not there is an etiological factor involved in the refractoriness of large ovarian follicles to hCG during pregnancy should be investigated.
REFERENCES
- 1. Burdick HO, Cimpa V. Further observations on induced ovulation in mice: a refractory period in early pregnancy. Endocrinology 1944; 35: 473–476. [Google Scholar]
- 2. Rowlands IW. The corpus luteum of the guinea pig. Ciba Foundation Colloquia Aging 1956; 2: 69–85. [Google Scholar]
- 3. Greenwald GS, Choudary JB. Follicular development and induction of ovulation in the pregnant mouse. Endocrinology 1969; 84: 1512–1516. [DOI] [PubMed] [Google Scholar]
- 4. Greenwald GS. Ovarian follicular development and pituitary FSH and LH content in the pregnant rat. Endocrinology 1966; 79: 572–578. [DOI] [PubMed] [Google Scholar]
- 5. Kagabu S. The influence of age on the number of non‐atretic follicles classified according to follicular size in the rat ovary. Exp Anim 1986; 35: 165–167. [DOI] [PubMed] [Google Scholar]
- 6. Braw RH, Tsafriri A. Effect of PMSG on follicular atresia in the immature rat ovary. J Reprod Fertil 1980; 59: 267–272. [DOI] [PubMed] [Google Scholar]
- 7. Kagabu S, Mamba K, Umezu M. Concentration of ovulatory LH in rats sera treated with PMSG and effect of small dose of hCG on follicular atresia. Jpn J Fertil Steril 1995; 40: 326–330. [Google Scholar]
- 8. Singh KB, Greenwald GS. Effects of continuous light on the reproductive cycle of the female rat: induction of ovulation and pituitary gonadotrophins during persistent oestrus. J Endocrinol 1967; 38: 389–394. [DOI] [PubMed] [Google Scholar]


