Abstract
Aim: Endometrial tissue inhibitors of metalloproteinases (TIMPs) appear to play an essential role during early implantation by modulating the invasiveness of the trophoblast. The expression of TIMP‐1, TIMP‐2 and TIMP‐3 in human endometrial stromal cells (ESC) was investigated during decidualization in vitro.
Methods: Endometrial stromal cells were isolated from hysterectomy specimens from premenopausal women undergoing surgery for benign reasons. Decidualization in vitro was induced by the application of 1 µmol/L progesterone and 30 nmol/L 17β‐estradiol over 9 days. The expression of TIMP‐1, TIMP‐2 and TIMP‐3 in ESC was measured by semiquantitative real‐time reverse transcription polymerase chain reaction and enzyme‐linked immunosorbent assay over intervals of 3 days.
Results: Decidualization in vitro was confirmed by a significant increase in prolactin expression. TIMP‐1 and TIMP‐2 mRNA and secreted protein showed no significant changes over the time‐course of decidualization. In contrast, TIMP‐3 was upregulated during the first 3 days of decidualization. An eightfold upregulation was observed until day 6, and the effect was less pronounced by day 9.
Conclusion: These results suggest a regulatory role of the TIMP system for endometrial differentiation in the second half of the menstrual cycle and in early implantation. The expression pattern of endometrial TIMP‐3 might be important for the regulation of trophoblast invasion. (Reprod Med Biol 2008; 7: 169–175)
Keywords: decidualization, endometrium, expression, implantation, tissue inhibitors of metalloproteinases
INTRODUCTION
DECIDUALIZATION OF THE stromal layer of the human endometrium in the second half of the menstrual cycle is a prerequisite for successful implantation of an embryo. 1 , 2 , 3 , 4 This complex process, which includes biochemical and morphological differentiation of the stromal cells, is mainly under the control of the ovarian steroid hormones estrogen and progesterone, which prepare the endometrium for the narrow time frame of receptivity, also known as the ‘window of implantation’. 4 , 5 , 6 The specific temporal and spatial expression patterns of various growth factors, cytokines, receptors and other signaling molecules are thought to play an important role in endometrial preparation for implantation. 4 In contrast, disturbed endometrial expression of these factors appears to play an essential role in a significant number of infertile patients.
The invasion of the trophoblast into the maternal endometrial stroma is a highly regulated process and essential for successful implantation and placentation. 1 , 2 , 7 The transiently invasive property of trophoblastic cells is characterized by the spatiotemporal expression of matrix metalloproteinases (MMPs). 8 , 9 MMP‐1, MMP‐2 and MMP‐9 are considered to be the most important proteases in extracellular matrix (ECM) remodelling during trophoblastic invasion. 10 , 11 , 12 In contrast to tumor invasion, trophoblastic invasion into the maternal endometrium is a highly coordinated and limited process, requiring a subtle interaction between the trophoblast and the endometrium. 13
Tissue inhibitors of metalloproteinases (TIMPs) are specific regulators of MMPs. 14 TIMPs inhibit the activity of MMPs by binding to their active sites in a 1:1 ratio. Four members of the mammalian TIMP family have been identified to date, and TIMP‐1, TIMP‐2 and TIMP‐3 have been characterized best. 14 Despite amino acid homology, each TIMP has an individual inhibitory profile of MMPs. TIMP‐1 preferentially binds to MMP‐9, whereas TIMP‐2 has a high affinity for MMP‐2. 9 TIMP‐3 has not as well characterized, but it has been shown to regulate MMP activity. 14 , 15 Membrane‐type MMPs are particularly sensitive to TIMP‐2 and TIMP‐3.
Members of the TIMP family expressed in the human endometrium during implantation represent a substantial regulatory barrier to invasion. 16 The importance of the endometrial TIMPs is further supported by the observation of decreased TIMP‐3 mRNA levels in the mid‐secretory endometrium of patients with unexplained infertility and/or recurrent miscarriages compared with normal mid‐secretory endometrium. 17 Although several studies have reported the expression of TIMPs in the human endometrium in vivo, 17 , 18 , 19 , 20 , 21 several discrepancies occur between the observed expression patterns of endometrial TIMPs.
Therefore, the aim of the present study was to analyze the expression patterns of TIMP‐1, TIMP‐2 and TIMP‐3 in human endometrial stromal cells during decidualization in vitro.
MATERIALS AND METHODS
Isolation and culture of human endometrial stromal cells
AFTER APPROVAL BY the local ethics board, endometrial tissue was obtained with informed consent from premenopausal women undergoing hysterectomy for benign reasons. All patients had regular menstrual cycles and were considered to be healthy with the exception of intramural uterine leiomyoma. Patients suffering from endometriosis, endometrial hyperplasia and endometrial polyps were not included in the present study. Tissue samples were washed with phosphate‐buffered saline (PBS) twice, minced into small pieces and digested for 1 h at 37°C in 0.5% collagenase (200 IU/mg; Biochrom, Berlin, Germany) in DMEM/F‐12 medium without phenol red (Gibco, Karlsruhe, Germany). Separation of endometrial stromal cells (ESC) was carried out by filtration through a 180 µm nylon membrane (Millipore, Bedford, MA, USA) followed by a 40 µm nylon sieve (Falcon, Heidelberg, Germany). The ESC that passed through the 40 µm sieve were thoroughly washed with PBS, seeded in 75 cm2 culture flasks and incubated in DMEM/F‐12 without phenol red containing 10% charcoal‐stripped fetal bovine serum (Biochrom) and 1% gentamycin (Cambrex, Walkersville, MD, USA).
Validation of cell culture purity
The purity of the ESC cultures was tested by flow‐cytometric analysis of vimentin (to detect ESC) and cytokeratin (to exclude epithelial cells) expression. For intracellular staining of vimentin and cytokeratin, the cells were permeabilized and fixed using fluorescent activated cell sorting Permeabilizing Solution and CellFIX (both from BD Biosciences, Heidelberg, Germany) following a standard protocol. The samples were stained using fluorescein‐isothiocyanate‐conjugated antivimentin and R‐phycoerythrin‐conjugated anticytokeratin mouse monoclonal antibodies (both from Santa Cruz Biotechnology, Santa Cruz, CA, USA) and were analyzed on a FACSCalibur cytometer using Cell Quest Pro software (BD Biosciences). Each staining sample was accompanied by a parallel staining with a corresponding unspecific mouse IgG1 isotype control (Acris, Hiddenhausen, Germany).
Decidualization of endometrial stromal cells in vitro
After reaching approximately 80% of confluence, cells were gently detached with trypsin and seeded into 24‐well culture plates at a density of 1 × 105 cells/well and the experiments were carried out in quadruplicate. For all experiments ESC were used from a single separation and samples from different patients were not pooled. In the present study, ESC were passaged only once because a reduced potential to decidualize after several passages has been shown. 22 Decidualization in vitro was induced by incubating the cells in culture medium containing 30 nmol/L 17β‐estradiol and 1 µmol/L progesterone (both from Sigma, Taufkirchen, Germany) for 9 days. 23 Decidualization was demonstrated by recording a significant increase in prolactin mRNA. At days 0, 3, 6 and 9 during the time‐course of decidualization, supernatants were collected and stored at –20°C until assayed and cells were detached with trypsin to be used for total RNA extraction.
RNA extraction and semiquantitative real‐time reverse transcription polymerase chain reaction
Total RNA was isolated from ESC using Trizol (Invitrogen, Karlsruhe, Germany) following the manufacturer's protocol. The purity and yield of the RNA were assessed spectrophotometrically. Total RNA was reverse‐transcribed using the High Capacity cDNA Archive Kit from Applied Biosystems (Foster City, CA, USA) according to the manufacturer's instructions. Semiquantitative real‐time polymerase chain reaction (PCR) was carried out to quantify mRNA levels of prolactin, TIMP‐1, TIMP‐2 and TIMP‐3 in relation to the housekeeping gene β‐actin. cDNA samples were amplified in a volume of 10 µL containing 1× SYBR Green PCR‐Master Mix (Applied Biosystems) and the respective forward and reverse primers (300 nmol/L). The primers (Invitrogen) were designed using Primer Express Primer Design Software v2.0 (Applied Biosystems) and the resulting amplicons had an intron‐overlapping sequence. The sequences of the primers used were: β‐actin forward 5′‐CCTGGCACCCAGCAC‐AAT‐3′, β‐actin reverse 5′‐GCCGATCCACACGGAGTACT‐3′, PRL forward 5′‐CACCCCCGAAGACAAGGAG‐3′, PRL reverse 5′‐CCAGGATCGCAATATGCTGAC‐3′, TIMP‐1 forward 5′‐CAATTCCGACCTCGTCATCAG‐3′, TIMP‐1 reverse 5′‐CGCTGGTATAAGGTGGTCTGGT‐3′, TIMP‐2 forward 5′‐GAAACGACATTTATGGCAACCC‐3′, TIMP‐2 reverse 5′‐TTCTCAGGCCCTTTGAACATCT‐3′, TIMP‐3 forward 5′‐CTGCTGACAGGTCGCGTCTAT‐3′ and TIMP‐3 reverse 5′‐AGCTGGTCCCACCTCTCCAC‐3′.
The PCR amplification was carried out in duplicate in an ABI Prism 7000 sequence detector (Applied Biosystems) using the following cycling program: 2 min at 50°C, 10 min at 95°C, followed by 15 s at 95°C and 1 min at 60°C for a total of 40 cycles. The PCR products were analyzed by thermal dissociation to verify that a single specific PCR product had been amplified. Relative expression levels of PRL, TIMP‐1, TIMP‐2 and TIMP‐3 in relation to the reference gene β‐actin were determined using the mathematical model ratio = 2−ΔΔCT. 24
Enzyme‐linked immunosorbent assays for TIMP‐1 and TIMP‐2
TIMP‐1 and TIMP‐2 in cell‐culture supernatants from ESC were determined using commercially available enzyme‐linked immunosorbent assay kits (R & D Systems, Wiesbaden, Germany) with a sensitivity of 0.08 ng/mL for TIMP‐1 and 0.06 ng/mL for TIMP‐2. There was no significant cross‐reactivity or interference and the intra‐assay and interassay variability were lower than 5%. Both assays were carried out according to the manufacturer's instructions and all samples were measured in duplicate.
Statistical analysis
Each experiment was carried out in triplicate or quadruplicate on cell cultures derived from three to five different patients. The data were analysed using one‐way anovas, followed by Dunnett's and Bonferroni multiple comparison tests using GraphPad PRISM version 4 software (GraphPad, San Diego, CA, USA). The results are expressed as mean ± standard error of the mean (SEM). Differences were considered to be significant at P < 0.05.
RESULTS
VALIDATION OF CELL culture purity using flow cytometric analysis of intracellular vimentin showed that more than 95% of the cultured cells were ESC (Fig. 1a). Cytokeratin staining confirmed the presence of less than 5% epithelial cells in the cell cultures used in the present study (data not shown). Figure 1b shows a continuous increase of PRL mRNA in human ESC during the time‐course of decidualization in vitro that was statistically significant at days 6 and 9. This expression pattern of the typical decidualization marker PRL confirmed the functionality of the protocol using 1 µmol/L progesterone and 30 nmol/L 17β‐estradiol over 9 days to decidualize ESC in vitro. During decidualization in vitro, TIMP‐1 and TIMP‐2 mRNA levels in ESC showed no significant changes (Fig. 2a,b). Nearly identical results were observed for the secretion of TIMP‐1 and TIMP‐2 protein from ESC (Fig. 3a,b). In contrast, a rapid and significant increase in TIMP‐3 mRNA levels in ESC between days 0 and 3 was observed (Fig. 2c). At day 6 TIMP‐3 levels were still strongly elevated, but from day 6 to day 9 the expression of TIMP‐3 declined significantly (Fig. 2c). In non‐decidualized ESC cultured in parallel over 9 days (without adding 17β‐estradiol and progesterone) there were no significant changes in TIMP‐3 during the time‐course (Fig. 2c).
Figure 1.
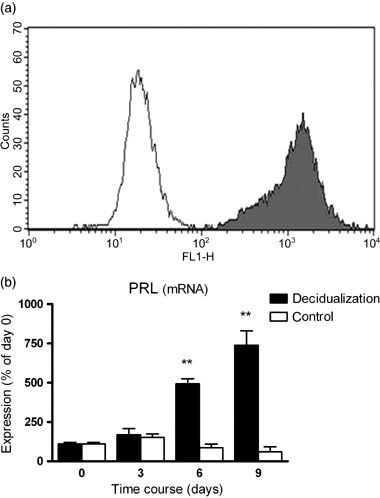
(a) Endometrial stromal cells (ESC) were stained intracellularly for vimentin and analyzed by flow cytometry (gray shading, vimentin staining; no shading, isotype control). One typical result is shown. (b) Levels of prolactin mRNA in ESC during decidualization in vitro. Filled bars represent ESC treated with 1 µmol/L progesterone and 30 nmol/L 17β‐estradiol and the open bars represent cells without hormonal treatment. Results are standardized relative to the levels on day 0, which were set at 100%. Bars represent mean ± standard error of the mean. **P < 0.01 compared to day 0. One typical result is shown.
Figure 2.
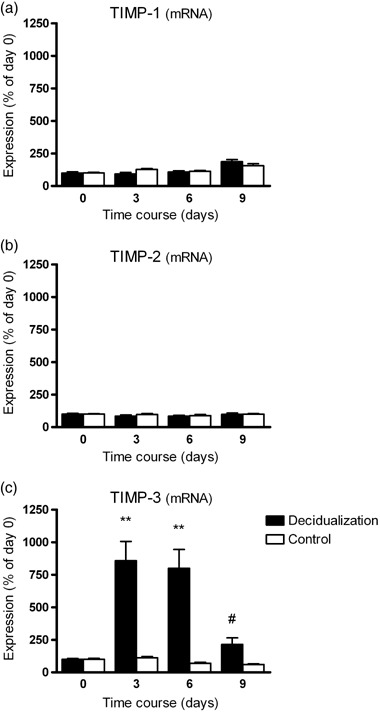
Levels of (a) tissue inhibitor of metalloproteinase‐1 (TIMP‐1), (b) TIMP‐2 and (c) TIMP‐3 mRNA in endometrial stromal cells (ESC) during decidualization in vitro. Filled bars represent ESC treated with 1 µmol/L progesterone and 30 nmol/L 17β‐estradiol and the open bars represent cells without hormonal treatment. Results are standardized relative to the levels on day 0, which were set at 100%. Bars represent mean ± standard error of the mean (n = 5). **P < 0.01 compared to day 0 and untreated controls; #P < 0.01 day 9 versus day 6.
Figure 3.
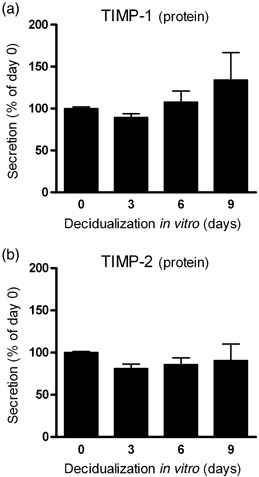
Levels of (a) tissue inhibitor of metalloproteinase‐1 (TIMP‐1) and (b) TIMP‐2 protein secretion by endometrial stromal cells (ESC) during decidualization in vitro. Bars represent ESC treated with 1 µmol/L progesterone and 30 nmol/L 17β‐estradiol. Results are standardized relative to the levels on day 0, which were set at 100%. Bars represent mean ± standard error of the mean (n = 3).
DISCUSSION
THE ENDOMETRIAL MMP/TIMP system has been shown to be involved in physiological and pathological extracellular matrix (ECM) remodelling. 9 The coordinated expression and interaction of these proteases and their specific inhibitors is necessary for successful decidualization of the endometrial stroma in the secretory phase as well as for the process of tissue degradation during menstruation in a non‐conceptive cycle. 9 , 13 In addition, during the complex process of implantation, trophoblastic MMPs and endometrial TIMPs have a key function in regulating the depth of invasion. 1 , 10 , 25 Moreover, a well‐structured ECM around the ESC is an essential prerequisite for successful implantation. 1 , 26
TIMP‐1, TIMP‐2 and TIMP‐3 have been identified in the human endometrium in vivo. 20 Using conventional RT‐PCR, Goffin and coworkers detected TIMP‐1, TIMP‐2 and TIMP‐3 throughout the cycle and observed no significant cyclic changes in TIMP‐1 and TIMP‐2. 19 In contrast, TIMP‐3 mRNA levels increased during the late secretory and menstrual phase compared with the proliferative and early secretory phase. 19 Another study using northern blot analysis describes significantly higher levels of TIMP‐3 in mid‐secretory phase endometrium than in proliferative endometrium. 17 A similar result was observed by Higuchi and colleagues using in situ hybridization, who reported stromal TIMP‐3 expression during the mid to late secretory phase. 18 Immunohistochemistry analysis indicated that TIMP‐3 expression peaks during the early to mid‐luteal phase. 27 Despite the small discrepancies between these studies, endometrial TIMP‐3 appears to be upregulated in the secretory phase, whereas TIMP‐1 and TIMP‐2 show a stable expression pattern over the menstrual cycle.
Very little data is available on the regulation of TIMPs in endometrial stromal cells in vitro. One study examined the expression of TIMP‐3 in human ESC using northern blotting and described an upregulation of TIMP‐3 mRNA under the influence of progesterone and estradiol. 18 In contrast to the results presented here, these researchers observed a slow increase in TIMP‐3 beginning at day 6 and culminating at day 12; these different results might be explained by the different protocols used for decidualization in vitro. In accordance with our results, another study described an unchanged expression of TIMP‐1 mRNA in human ESC during decidualization; this study used different concentrations of hormones compared with the present study. 28 However, nothing is known about TIMP‐1 protein or TIMP‐2 expression in human ESC in vitro and the data on TIMP‐1 and TIMP‐3 in vitro are difficult to compare because of different experimental conditions.
This is the first study to systematically examine the expression of TIMP‐1, TIMP‐2 and TIMP‐3 in an often used cell‐culture model in vitro. Our findings in vitro are in accordance with the findings in vivo and further support the feasibility of this cell culture and decidualization model to mimic the secretory phase in vitro. The constant expression of TIMP‐1 and TIMP‐2 might be interpreted as a type of housekeeping function, whereas the strongly regulated TIMP‐3 appears to play a more specific role in the secretory endometrium.
Unfortunately, we could not detect TIMP‐3 protein in ESC in vitro. Attempts to detect TIMP‐3 using immunofluorescent staining of ESC monolayers and western blot analysis of ESC lysates or cell‐culture supernatants were not successful. Similar problems have been described by Salamonsen et al., who also failed to detect TIMP‐3 at the protein level in endometrial cells. 29 There is a report showing TIMP‐3 in cell‐culture supernatants of ESC, as detected by western blotting; 30 however, in this study a 20‐fold concentration of conditioned medium was necessary to detect TIMP‐3. In this context it should be mentioned that there is an important difference between TIMP‐1 and TIMP‐2 on the one hand and TIMP‐3 on the other. TIMP‐1 and TIMP‐2 are secreted and then released into the intercellular space or cell‐culture supernatant, whereas TIMP‐3 is rapidly bound to special components of the ECM. 9 As a result of this binding, TIMP‐3 has been suggested to play an additional regulatory role during the invasion processes. 31 These different properties of the TIMP molecules as well as the very low protein levels of TIMP‐3 might explain the difficulties in detecting this protein in the human endometrium.
The physiological impact of an unaltered expression of endometrial TIMPs on the early implantation process is not fully understood. Data obtained in vitro clearly point to a role as modulators of implantation for endometrial TIMPs. 26 , 32 In addition, TIMP‐3 is also secreted by the invading cytotrophoblast itself, suggesting an additional autocrine regulatory function of trophoblast invasion. 18 , 33 Interestingly, decreased levels of TIMP‐3 mRNA were observed in the mid‐secretory endometrium of patients with unexplained infertility or recurrent miscarriages compared with normal mid‐secretory endometrium, 17 suggesting an impact of altered endometrial TIMP‐3 on implantation.
Apart from counteracting MMPs, several additional functions have been attributed to individual TIMPs. One interesting feature of TIMP‐3 and TIMP‐2 is their ability to induce apoptosis in different cell types. 34 , 35 , 36 , 37 Interestingly, direct anti‐apoptotic and proliferating effects have been observed for TIMP‐1. 38 , 39 , 40 The complex role of TIMPs in the cycling human endometrium and at the feto–maternal interface remains to be elucidated and the various functions of TIMPs need to be considered in further investigations.
In summary, the observed expression patterns of TIMP‐1, TIMP‐2 and TIMP‐3 in human ESC during decidualization in vitro strongly resemble the situation in vivo. The selective regulation of TIMP‐3 in human ESC during decidualization further underlines the important role that this molecule might play in endometrial differentiation and in the process of early implantation. The pronounced upregulation of TIMP‐3 at the maternal implantation site might be essential for the final preparation of a receptive endometrium and for directly modulating the first invasion steps of the implanting embryo.
ACKNOWLEDGMENTS
WE GRATEFULLY APPRECIATE the collaboration with Sebastian Wesselborg (Department of Internal Medicine, University of Tübingen, Germany), who allowed us to use the ABI Prism 7000 sequence detector to carry out the real‐time RT‐PCR measurements.
REFERENCES
- 1. Aplin JD. Implantation, trophoblast differentiation and haemochorial placentation: mechanistic evidence in vivo and in vitro . J Cell Sci 1991; 99: 681–692. [DOI] [PubMed] [Google Scholar]
- 2. Bischof P, Meisser A, Campana A. Biochemistry and molecular biology of trophoblast invasion. Ann NY Acad Sci 2001; 943: 157–162. [DOI] [PubMed] [Google Scholar]
- 3. Dunn CL, Kelly RW, Critchley HO. Decidualization of the human endometrial stromal cell: an enigmatic transformation. Reprod Biomed Online 2003; 7: 151–161. [DOI] [PubMed] [Google Scholar]
- 4. Strowitzki T, Germeyer A, Popovici R, Von Wolff M. The human endometrium as a fertility‐determining factor. Hum Reprod Update 2006; 12: 617–630. [DOI] [PubMed] [Google Scholar]
- 5. Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med 1999; 340: 1796–1799. [DOI] [PubMed] [Google Scholar]
- 6. Irwin JC, Utian WH, Eckert RL. Sex steroids and growth factors differentially regulate the growth and differentiation of cultured human endometrial stromal cells. Endocrinology 1991; 129: 2385–2392. [DOI] [PubMed] [Google Scholar]
- 7. Pijnenborg R, Dixon G, Robertson WB, Brosens I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta 1980; 1: 3–19. [DOI] [PubMed] [Google Scholar]
- 8. Bischof P, Campana A. Molecular mediators of implantation. Baillieres Best Pract Res Clin Obstet Gynaecol 2000; 14: 801–814. [DOI] [PubMed] [Google Scholar]
- 9. Curry TE Jr, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev 2003; 24: 428–465. [DOI] [PubMed] [Google Scholar]
- 10. Bischof P, Martelli M, Campana A, Itoh Y, Ogata Y, Nagase H. Importance of matrix metalloproteinases in human trophoblast invasion. Early Pregnancy 1995; 1: 263–269. [PubMed] [Google Scholar]
- 11. Librach CL, Werb Z, Fitzgerald ML et al 92‐kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol 1991; 113: 437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shimonovitz S, Hurwitz A, Dushnik M, Anteby E, Geva‐Eldar T, Yagel S. Developmental regulation of the expression of 72 and 92 kd type IV collagenases in human trophoblasts: a possible mechanism for control of trophoblast invasion. Am J Obstet Gynecol 1994; 171: 832–838. [DOI] [PubMed] [Google Scholar]
- 13. Hulboy DL, Rudolph LA, Matrisian LM. Matrix metalloproteinases as mediators of reproductive function. Mol Hum Reprod 1997; 3: 27–45. [DOI] [PubMed] [Google Scholar]
- 14. Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol 1997; 74: 111–122. [PubMed] [Google Scholar]
- 15. Birkedal‐Hansen H, Moore WG, Bodden MK et al Matrix metalloproteinases: a review. Crit Rev Oral Biol Medical 1993; 4: 197–250. [DOI] [PubMed] [Google Scholar]
- 16. Zhang J, Salamonsen LA. Tissue inhibitor of metalloproteinases (TIMP)‐1, ‐2 and ‐3 in human endometrium during the menstrual cycle. Mol Hum Reprod 1997; 3: 735–741. [DOI] [PubMed] [Google Scholar]
- 17. Jokimaa V, Oksjoki S, Kujari H, Vuorio E, Anttila L. Altered expression of genes involved in the production and degradation of endometrial extracellular matrix in patients with unexplained infertility and recurrent miscarriages. Mol Hum Reprod 2002; 8: 1111–1116. [DOI] [PubMed] [Google Scholar]
- 18. Higuchi T, Kanzaki H, Nakayama H et al Induction of tissue inhibitor of metalloproteinase 3 gene expression during in vitro decidualization of human endometrial stromal cells. Endocrinology 1995; 136: 4973–4981. [DOI] [PubMed] [Google Scholar]
- 19. Goffin F, Munaut C, Frankenne F et al Expression pattern of metalloproteinases and tissue inhibitors of matrix‐metalloproteinases in cycling human endometrium. Biol Reprod 2003; 69: 976–984. [DOI] [PubMed] [Google Scholar]
- 20. Henriet P, Cornet PB, Lemoine P et al Circulating ovarian steroids and endometrial matrix metalloproteinases (MMPs). Ann NY Acad Sci 2002; 955: 119–138. [DOI] [PubMed] [Google Scholar]
- 21. Vassilev V, Pretto CM, Cornet PB et al Response of matrix metalloproteinases and tissue inhibitors of metalloproteinases messenger ribonucleic acids to ovarian steroids in human endometrial explants mimics their gene‐ and phase‐specific differential control in vivo. J Clin Endocrinol Metab 2005; 90: 5848–5857. [DOI] [PubMed] [Google Scholar]
- 22. Von Wolff M, Stieger S, Lumpp K, Bucking J, Strowitzki T, Thaler CJ. Endometrial interleukin‐6 in vitro is not regulated directly by female steroid hormones, but by pro‐inflammatory cytokines and hypoxia. Mol Hum Reprod 2002; 8: 1096–1102. [DOI] [PubMed] [Google Scholar]
- 23. Fluhr H, Krenzer S, Deperschmidt M, Zwirner M, Wallwiener D, Licht P. Human chorionic gonadotropin inhibits insulin‐like growth‐factor‐binding protein‐1 and prolactin in decidualized human endometrial stromal cells. Fertil Steril 2006; 86: 236–238. [DOI] [PubMed] [Google Scholar]
- 24. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2‐Delta Delta C(T) method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 25. Bischof P, Meisser A, Campana A. Paracrine and autocrine regulators of trophoblast invasion – a review. Placenta 2000; 21: S55–S60. [DOI] [PubMed] [Google Scholar]
- 26. Bischof P, Campana A. A model for implantation of the human blastocyst and early placentation. Hum Reprod Update 1996; 2: 262–270. [DOI] [PubMed] [Google Scholar]
- 27. Chegini N, Rhoton‐Vlasak A, Williams RS. Expression of matrix metalloproteinase‐26 and tissue inhibitor of matrix metalloproteinase‐3 and ‐4 in endometrium throughout the normal menstrual cycle and alteration in users of levonorgestrel implants who experience irregular uterine bleeding. Fertil Steril 2003; 80: 564–570. [DOI] [PubMed] [Google Scholar]
- 28. Lockwood CJ, Krikun G, Hausknecht VA, Papp C, Schatz F. Matrix metalloproteinase and matrix metalloproteinase inhibitor expression in endometrial stromal cells during progestin‐initiated decidualization and menstruation‐related progestin withdrawal. Endocrinology 1998; 139: 4607–4613. [DOI] [PubMed] [Google Scholar]
- 29. Salamonsen LA, Butt AR, Hammond FR, Garcia S, Zhang J. Production of endometrial matrix metalloproteinases, but not their tissue inhibitors, is modulated by progesterone withdrawal in an in vitro model for menstruation. J Clin Endocrinol Metab 1997; 82: 1409–1415. [DOI] [PubMed] [Google Scholar]
- 30. Raga F, Casan EM, Wen Y, Huang HY, Bonilla‐Musoles F, Polan ML. Independent regulation of matrix metalloproteinase‐9, tissue inhibitor of metalloproteinase‐1 (TIMP‐1), and TIMP‐3 in human endometrial stromal cells by gonadotropin‐releasing hormone: implications in early human implantation. J Clin Endocrinol Metab 1999; 84: 636–642. [DOI] [PubMed] [Google Scholar]
- 31. Pavloff N, Staskus PW, Kishnani NS, Hawkes SP. A new inhibitor of metalloproteinases from chicken: ChIMP‐3. A third member of the TIMP family. J Biol Chem 1992; 267: 17 321–17 326. [PubMed] [Google Scholar]
- 32. Pijnenborg R, Robertson WB, Brosens I, Dixon G. Review article. Trophoblast invasion and the establishment of haemochorial placentation in man and laboratory animals. Placenta 1981; 2: 71–91. [DOI] [PubMed] [Google Scholar]
- 33. Bass KE, Li H, Hawkes SP et al Tissue inhibitor of metalloproteinase‐3 expression is upregulated during human cytotrophoblast invasion in vitro. Dev Genet 1997; 21: 61–67. [DOI] [PubMed] [Google Scholar]
- 34. Baker AH, Zaltsman AB, George SJ, Newby AC. Divergent effects of tissue inhibitor of metalloproteinase‐1, ‐2, or ‐3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. TIMP‐3 promotes apoptosis. J Clin Invest 1998; 101: 1478–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baker AH, George SJ, Zaltsman AB, Murphy G, Newby AC. Inhibition of invasion and induction of apoptotic cell death of cancer cell lines by overexpression of TIMP‐3. Br J Cancer 1999; 79: 1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bond M, Murphy G, Bennett MR, Newby AC, Baker AH. Tissue inhibitor of metalloproteinase‐3 induces a Fas‐associated death domain‐dependent type II apoptotic pathway. J Biol Chem 2002; 277: 13 787–13 795. [DOI] [PubMed] [Google Scholar]
- 37. Lim MS, Guedez L, Stetler‐Stevenson WG, Stetler‐Stevenson M. Tissue inhibitor of metalloproteinase‐2 induces apoptosis in human T lymphocytes. Ann NY Acad Sci 1999; 878: 522–523. [DOI] [PubMed] [Google Scholar]
- 38. Boulday G, Fitau J, Coupel S, Soulillou JP, Charreau B. Exogenous tissue inhibitor of metalloproteinase‐1 promotes endothelial cell survival through activation of the phosphatidylinositol 3‐kinase/Akt pathway. Ann NY Acad Sci 2004; 1030: 28–36. [DOI] [PubMed] [Google Scholar]
- 39. Chromek M, Tullus K, Lundahl J, Brauner A. Tissue inhibitor of metalloproteinase 1 activates normal human granulocytes, protects them from apoptosis, and blocks their transmigration during inflammation. Infect Immun 2004; 72: 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu XW, Taube ME, Jung KK et al Tissue inhibitor of metalloproteinase‐1 protects human breast epithelial cells from extrinsic cell death: a potential oncogenic activity of tissue inhibitor of metalloproteinase‐1. Cancer Res 2005; 65: 898–906. [PubMed] [Google Scholar]


