Fig. 2.
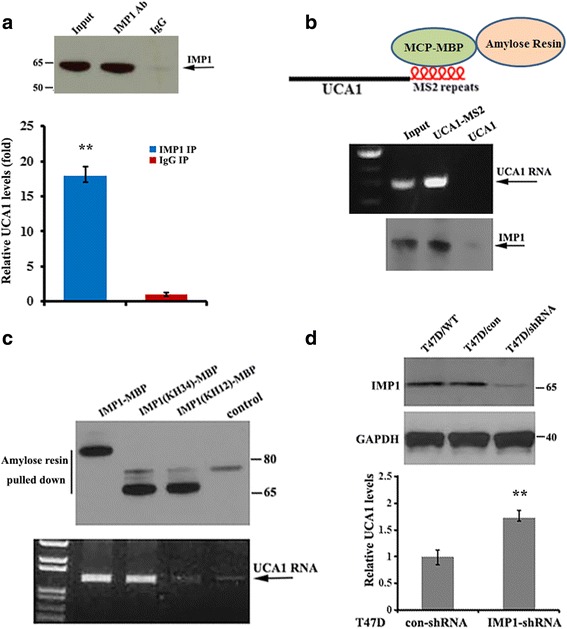
Interaction of insulin-like growth factor 2 messenger RNA binding protein (IMP1) with urethral carcinoma-associated 1 (UCA1) in breast cancer cells. a Upper: representitive western blots indicate the precipitation (IP) of IMP1 in T47D cells from co-IP experiments; lower: RT-qPCR shows that UCA1 was preferentially co-precipitated with IMP1 antibody relative to the IgG control. Data are normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) messenger RNA (mRNA) and statistically analyzed: **P < 0.01. b Upper: shows the UCA1-MS2 chimeric RNA, which can be bound by MBP-MCP. Lower: UCA1-MS2 was pulled down by MBP-MCP conjugated to amylose resin. Co-precipitated UCA1-MS2 and IMP1 are shown by RT-PCR and western blot analyses. c Empty vector (control) and vectors expressing MBP-fused full-length IMP1, IMP1(KH34) or IMP1(KH12) were transiently transfected into 293 T cells. Pulldown assays with amylose resin and western blotting were performed to detect co-precipitation of IMP1 truncates with UCA1. Results show that the full-length IMP1 and IMP1(KH34) co-precipitated with UCA1 in the cell extracts. d Knockdown of IMP1 in T47D cells increased stability of UCA1. Upper: immunoblots showing expression of IMP1 in T47D/IMP1-shRNA and control cells. Lower: RT-qPCR indicating levels of UCA1 in IMP1-downregulated cells. Relative levels of UCA1 RNA are normalized to GAPDH mRNA and the data are presented as means ± SD from three independent experiments: **P < 0.01. **P < 0.01 as determined by Student’s t test
