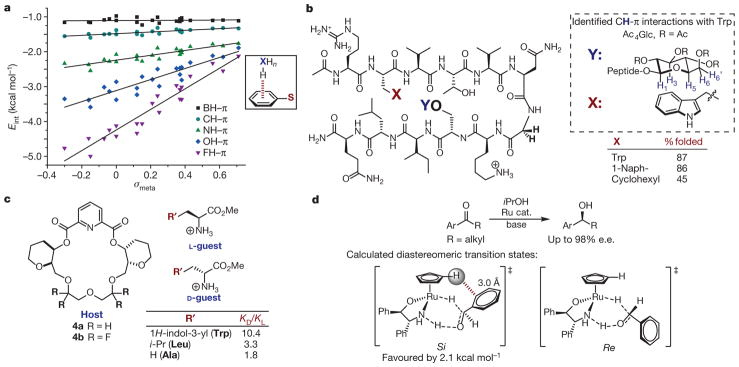
Figure 4. Studies of XH–π interactions.
a, Effect of X and arene identity on XH–π interactions. The structure that was computed to determine Eint in each case is shown boxed at right; S is a variable substituent. Adapted from ref. 34, American Chemical Society. b, Double mutant cycles in β-hairpin oligopeptide to quantify CH–π interactions. Left, the oligopeptide under evaluation, where X and Y are variable substituents. Previous studies support the CH–π interactions as shown in the box on the right. c, Chiral host (left) binds amino acid guests (right) selectively through a CH–π interaction, as measured by KD/KL. d, Rationalization of enantioselectivity in asymmetric transfer hydrogenation of aryl ketones. Top, reaction studied; bottom, calculated diastereomeric transition states (Si and Re isomers shown left and right, respectively). e.e., enantiomeric excess.