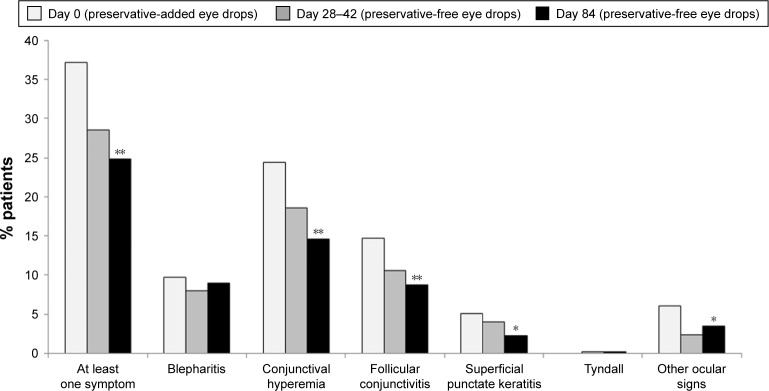
Figure 6.
Reduction in clinical signs following switch from preservative-added to preservative-free eye drops.
Notes: In an open, prospective clinical trial, 435 glaucoma patients were switched from their previous preservative-added timolol medication to a preservative-free timolol preparation. Clinical signs were recorded during 84 days of treatment (*p<0.01, **p<0.05). Reproduced from Bron A, Chiambaretta F, Pouliquen P, Rigal D, Rouland JF. Efficacy and safety of substituting a twice-daily regimen of timolol with a single daily instillation of nonpreserved beta-blocker in patients with chronic glaucoma or ocular hypertension. Français D’Ophtalmologie 2003; volume 26, issue 7:pages 668–674. Copyright © 2003, Elsevier Masson SAS. All rights reserved.26