Abstract
Background
Outpatient parenteral antimicrobial therapy (OPAT) is overused in cases where highly bioavailable oral alternatives would be equally effective. However, the scope of OPAT use for children nationwide is poorly understood. Our objective was to characterize OPAT use and clinical outcomes for a large population of pediatric Medicaid enrollees treated with OPAT.
Methods
We analyzed the Truven MarketScan Medicaid claims database between 2009 and 2012. An OPAT episode was identified by capturing children with claims data indicating home infusion therapy for an intravenous antimicrobial. We characterized OPAT use by describing patient demographics, diagnoses, and antimicrobials prescribed. We categorized an antimicrobial as highly bioavailable if ≥80% systemic exposure was expected from the peroral dose. We also determined the percentage of OPAT recipients in whom a follow-up healthcare encounter occurred during the OPAT episode in either the emergency department or as a hospital admission. We reviewed the primary diagnoses associated with these healthcare encounters to determine whether it was related to OPAT.
Results
We identified 3433 OPAT episodes in 2687 patients. A total of 4774 antimicrobials were prescribed during these episodes. Ceftriaxone and vancomycin were the most commonly prescribed antimicrobials. Highly bioavailable antimicrobials accounted for 34% of antimicrobials used for OPAT. An emergency department visit or hospital admission occurred during 38% of OPAT episodes, among which 61% were OPAT-related.
Conclusions
The high rate of medical encounters associated with OPAT in this cohort and the common prescribing of highly bioavailable antimicrobials underscore the opportunities for antimicrobial stewardship of pediatric OPAT.
Keywords: outpatient parenteral antimicrobial therapy, pediatrics
Outpatient parenteral antimicrobial therapy (OPAT) is the administration of intravenous (IV) antimicrobial medications outside of the inpatient hospital setting. Outpatient parenteral antimicrobial therapy has been used for nearly 40 years to treat infections requiring long-term antimicrobial use [1]. When considered relative to prolonged hospitalization, it is both cost-effective and relatively safe, resulting in extensive use both in adult and pediatric medical care practices [2–5]. National registries serve as a useful source in describing OPAT use; however, these data often lack detailed information concerning OPAT in children [6, 7]. Data from individual pediatric hospitals have shown that OPAT is most frequently used to treat respiratory tract (including cystic fibrosis), musculoskeletal, bloodstream, intra-abdominal, skin and soft tissue, urinary tract, and central nervous system infections [1, 8].
Children receiving prolonged antimicrobial therapy, including OPAT, experience a high rate of adverse events [9]. Outpatient parenteral antimicrobial therapy is overused, especially for conditions where highly bioavailable oral alternatives would be equally effective such as acute osteomyelitis [10]. Overuse of OPAT exposes children and their families to excess costs and potentially avoidable catheter-related complications including infection and thrombosis [5, 11, 12]. Some of these complications may result in hospital readmission or other unplanned healthcare encounters including emergency department (ED) visits. The high rate of hospital readmission for patients treated with OPAT was recently highlighted in a study of adult patients, but similar data for pediatric OPAT are sparse [13].
The objectives of this study were to characterize the most commonly used antimicrobials for OPAT, the diagnoses treated with OPAT, and healthcare use likely attributable to an OPAT complication for a large population of US children.
METHODS
Data Source and Study Design
We analyzed data from the Truven MarketScan (Ann Arbor, Michigan) Medicaid claims database between 2009 and 2012. The MarketScan Medicaid Database contains the pooled healthcare experience of approximately 6 million Medicaid enrollees from multiple anonymous states. In 2009 the database included 9 states, and in 2010–2012 12 states were included. The database includes patient demographic and diagnostic information, data on inpatient services received and outpatient prescription drug claims, as well as information on enrollment, long-term care, and other medical care.
Study Population and Diagnostic Categories
We included all Medicaid claims for children 0–18 years of age. Outpatient claims with a healthcare common procedure coding system (HCPCS) code indicating home infusion therapy [HIT; (S9494, S9497, S9500–504)], a current procedural terminology (CPT) code indicating HIT (99601, 99602), or a HCPCS code indicating HIT supplies (A4220–223, A4246–247, E0776, E0779–781, E0791, S5497–498, S5501–502, S5517–518, S5520–523) were considered HIT claims. The days between the date service incurred on the initial HIT claim and the date service ended on the final HIT claim were used to identify a HIT episode. Home infusion therapy claims with a gap of less than 30 days were considered to be part of the same HIT episode; if a gap of 30 days or more existed between the date service ended on 1 HIT claim and the date service was incurred for a subsequent HIT claim, the episodes were considered separate. Home infusion therapy episodes were considered OPAT episodes if either of the following were identified between the starting date and ending date of a HIT episode: (1) a concomitant claim with a HCPCS code indicating IV antimicrobial (Supplemental Table 1) use or (2) a concomitant retail pharmacy claim indicating a fill for an IV antimicrobial. Finally, only OPAT episodes in which patients were continuously enrolled in Medicaid during the duration of the OPAT episode were considered for analysis.
All International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes associated with the OPAT episode were collected (Supplemental Table 2). The discharge diagnostic code occurring most frequently and concurrently during an OPAT episode was considered the primary diagnosis. If ≥2 diagnoses occurred the same number of times, all were included in the analysis resulting in greater than 1 diagnosis per episode. Nine hundred twenty-six unique primary ICD-9-CM codes were identified, and each diagnosis was then grouped into 1 of 15 broader diagnostic categories based on consensus by 2 authors (J. L. G. and A. L. H.): hematology/oncology (H/O), gastrointestinal/genitourinary (GI/GU), cystic fibrosis (CF), osteoarticular, pulmonary (excluding CF), central nervous system (CNS), skin and soft tissue infection (SSTI), bacteremia, endovascular/endocarditis, upper respiratory tract (URI), urinary tract infection, surgical site, specific pathogen, other, and unknown. The 15 diagnostic categories were created in part by adapting from a previous study [14]. The pathogen category was selected when the only available code was specific to an organism without additional codes providing further information (eg, 04112 for methicillin-resistant Staphylococcus aureus).
Antimicrobial Use
For each OPAT episode, we determined the antimicrobial(s) used. We defined highly bioavailable antimicrobials as those with ≥80% systemic exposure from peroral dose [15], including clindamycin, fluconazole, fluoroquinolones, linezolid, metronidazole, trimethoprim-sulfamethoxazole, and voriconazole. The identification of highly bioavailable agents is important because in some instances, these agents could be prescribed in oral formulation and avoid OPAT.
Emergency Department Visit or Inpatient Hospitalization During Outpatient Parenteral Antimicrobial Therapy
All hospital admissions and ED encounters occurring during an OPAT episode were identified. The ED count included only encounters in which the patient was discharged from the ED. Patients admitted as an inpatient from the ED were only counted as an inpatient encounter. The same method for diagnostic classification was applied for these ED and inpatient visits during OPAT episodes. The following ICD-9-CM codes were selected as diagnoses related to an OPAT complication: (1) infection due to central venous catheter (99931); (2) mechanical complication of other vascular device, implant, and graft (9961); (3) other complications due to other vascular device, implant, and graft (99674); or (4) fever (78060, 78061). Although there are multiple potential causes of fever unrelated to OPAT, all ED and hospital admission visits with a ICD-9-CM code for fever were considered a complication, because when a fever does occur in a patient with a central catheter, it is standard practice to obtain a blood culture, which may require an acute care visit and evaluation.
Data Analysis
We used descriptive statistics to characterize patient demographics, diagnoses, antimicrobials, and ED visits and hospital admissions during OPAT episodes. We determined the percentage of OPAT episodes that contained highly bioavailable antimicrobials overall and for selected diagnoses. In addition, we calculated the percentage of OPAT episodes that resulted in healthcare use as either a hospital admission or ED visit overall and for each diagnostic category, including those episodes where healthcare use was related to an OPAT complication. Categorical variables were described using frequencies and percentages; continuous variables were described using median and interquartile range. Any comparisons of categorical variables were made using a χ2 test for association. P values <.05 were considered statistically significant. All analyses were performed by using with SAS 9.3 (SAS Institute, Inc., Cary, North Carolina). The Children's Mercy Hospital Institutional Review Board deemed this study exempt.
RESULTS
Patient Characteristics and Diagnosis Associated With Outpatient Parenteral Antimicrobial Therapy
We identified 3433 distinct OPAT episodes for 2687 patients. Patient demographics are shown in Table 1. Children less than 1 year of age accounted for a smaller percentage of OPAT than other age groups (P < .001). Overall, 3118 (91%) of OPAT episodes had a single diagnosis, whereas 315 (9%) had 2 or more. The most common hospital discharge diagnosis categories associated with OPAT episodes were H/O (18%), GI/GU (17%), CF (13%), osteoarticular (10%), and pulmonary (10%) (Table 2).
Table 1.
Demographics of Children Prescribed OPAT
| Demographics | Total Patients N = 2687 (%) |
|---|---|
| Gender, %male | 1427 (53) |
| Age, median (IQR) | 7 (2, 14) |
| Age Group | |
| a. Less than 1 year | 213 (8) |
| b. 1–5 years | 816 (30) |
| c. 6–12 years | 863 (32) |
| d. 13+ years | 795 (30) |
| Race/Ethnicity | |
| a. Non-Hispanic White | 1467 (55) |
| b. Non-Hispanic Black | 490 (18) |
| c. Hispanic | 221 (8) |
| d. Other | 509 (19) |
| OPAT Episodes | |
| a. Single episode | 2277 (85) |
| b. Multiple episodes | 410 (15) |
| OPAT episodes, mean (SE) | 1.28 (0.02) |
Abbreviations: IQR, interquartile range; OPAT, outpatient parenteral antimicrobial therapy; SE, standard error.
Table 2.
Distribution of Primary Diagnoses Among All OPAT Episodes
| Diagnostic Categorya | Prevalence (%), N = 3433 | Most Common Antimicrobials Prescribed (%)b |
|---|---|---|
| Hematology/Oncology (H/O) | 615 (17.9) | Ceftriaxone (26) Fluconaozle (22) Vancomycin (22) Acyclovir (12) Cefepime (11) |
| Gastrointestinal/Genitourinary (GI/GU) | 583 (17.0) | Ceftriaxone (20) Metronidazole (17) Vancomycin (84) Fluconazole (13) Piperacillin/tazobactam (13) |
| Cystic Fibrosis (CF) | 439 (12.8) | Tobramycin (47) Ceftazidime (27) Vancomycin (24) Meropenem (21) Cefepime (15) |
| Other | 417 (12.1) | Ceftriaxone (26) Fluconazole (17) Vancomycin (14) Clindamycin (6) Meropenem (6) |
| Osteoarticular | 346 (10.1) | Ceftriaxone (25) Vancomycin (24) Clindamycin (20) Cefazolin (18) Nafcillin (5) |
| Pulmonary | 340 (9.9) | Ceftriaxone (32) Vancomycin (22) Cefepime (11) Clindamycin (9) Fluconazole (9) |
| Central Nervous System (CNS) | 264 (7.7) | Ceftriaxone (38) Vancomycin (19) Gentamicin (13) Fluconazole (8) Metronidazole (7) |
| Skin Soft Tissue Infection (SSTI) | 256 (7.5) | Vancomycin (29 Ceftriaxone (22) Clindamycin (14) Cefazolin (9) Meropenem (6) |
| Pathogen | 244 (7.1) | Vancomycin (23) Ceftriaxone (19) Clindamycin (8) Cefazolin (7) Meropenem (6) |
| Bacteremia | 202 (5.9) | Ceftriaxone (23) Vancomycin (16) Ampicillin (15) Gentamicin (13) Cefepime (8) |
| Vascular/Endocarditis | 168 (4.9) | Vancomycin (30) Ceftriaxone (23) Fluconazole (10) Clindamycin (8) Gentamicin (7) |
| Upper Respiratory Infection (URI) | 145 (4.2) | Ceftriaxone (47) Vancomycin (16) Cefepime (10) Fluconazole (8) Ceftazidime (6) |
| Urinary Tract Infection (UTI) | 134 (3.9) | Ceftriaxone (28) Cefepime (11) Gentamicin (10) Vancomycin (9) Fluconazole (7) |
| Surgical Site | 88 (2.6) | Vancomycin (25) Cefazolin (13) Ceftriaxone (13) Nafcillin (8) Ciprofloxacin (7) |
| Unknown | 12 (0.3) | Ceftriaxone (17) Gentamicin (17) Amikacin (8) Ampicillin/sulbactam (8) Aztreonam (8) |
Abbreviations: ICD-9, International Classification of Diseases, Ninth Revision; OPAT, outpatient parenteral antimicrobial therapy.
aRefer to Supplemental Table 2 for the categorization scheme for diagnostic conditions by ICD-9 code.
bBold denotes highly bioavailable.
Antimicrobials Prescribed During an Outpatient Parenteral Antimicrobial Therapy Episode
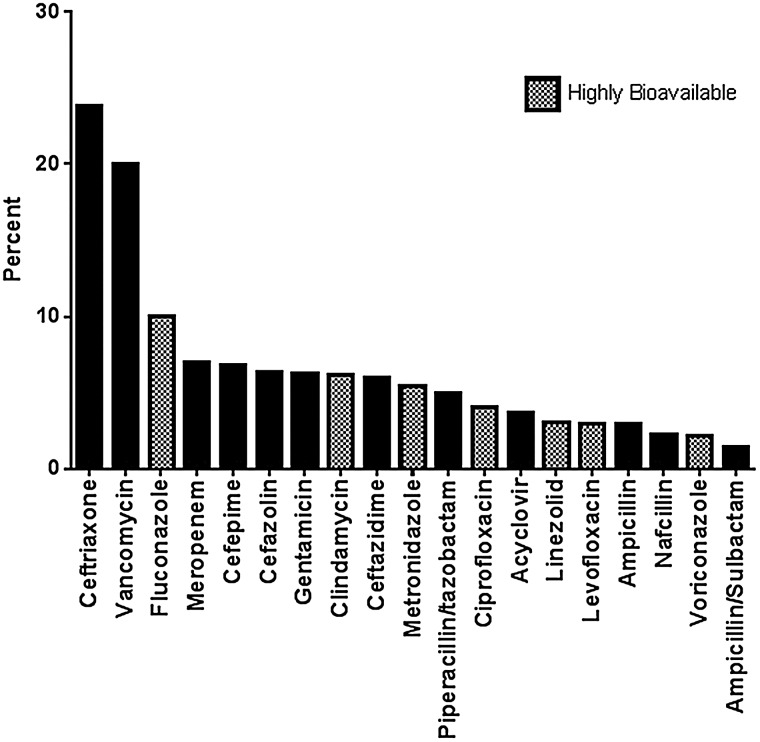
A total of 4774 antimicrobials were prescribed during the 3433 OPAT episodes. Ceftriaxone (24%) and vancomycin (20%) were the most commonly prescribed antimicrobials (Figure 1). Highly bioavailable antimicrobials accounted for 34% of overall antimicrobials prescribed during OPAT episodes, including 29% of OPAT episodes classified as osteoarticular and 31% classified as SSTI. The most frequently prescribed highly bioavailable antimicrobials were fluconazole (10% overall) and clindamycin (6% overall). Intravenous fluconazole was most commonly prescribed for H/O patients and for GI/GU and endovascular infections. Intravenous clindamycin was most commonly prescribed for osteoarticular infections and SSTIs.
Figure 1.
Frequency of antimicrobials prescribed during an outpatient parenteral antimicrobial therapy episode.
Healthcare Use During an Outpatient Parenteral Antimicrobial Therapy Episode
More than one third of children receiving OPAT (n = 1289; 38%) had either an ED visit or hospitalization during an OPAT episode (Table 3). Hematology/oncology diagnostic category was associated with the highest percentage of medical care encounters with 28% experiencing an ED visit and 51% having a hospitalization during an OPAT episode. Other categories with high rates of healthcare encounters were endovascular/endocarditis (27% ED, 24% hospitalization) and GI/GU (24% ED, 30% hospitalization). Overall, 61% of acute healthcare encounters during OPAT episodes were likely attributable to a catheter-related complication; this rate was relatively consistent across diagnostic categories (Table 3). Subanalysis was performed by including only diagnoses in which oral antimicrobial therapy would more commonly be considered (osteoarticular, pulmonary, SSTI, URI, renal, or surgical site), and 56% of healthcare encounters were due to OPAT complications. When applied to the entire cohort, 23% of OPAT episodes resulted in an ED visit or hospitalization related to an OPAT complication. Among children who experienced an OPAT-related complication, 25% were treated with a highly bioavailable antimicrobial. Of the 791 episodes of OPAT-related inpatient or ED use, 265 (33%) included ICD-9 code for fever, 276 (35%) included ICD-9 code for line complication, and 250 (32%) included ICD-9 codes for both fever and line complication.
Table 3.
Percentage of OPAT Episodes Involving at Least One Emergency Department Visit or Hospital Admission
| Diagnostic Catergorya | Overall ED Utilization by Children Receiving OPAT (%) | ED Utilization With OPAT Complicationb (%) | Overall Inpatient Utilization by Children Receiving OPAT (%) | Inpatient Utilization With OPAT Complicationb (%) | Overall Inpatient or ED Utilization by Children Receiving OPAT (%) | Inpatient or ED Utilization With OPAT Complicationb (%) |
|---|---|---|---|---|---|---|
| Hematology/Oncology (H/O), N = 615 | 174 (28) | 94 (54) | 311 (51) | 236 (76) | 368 (60) | 274 (75) |
| Gastrointestinal/Genitourinary (GI/GU), N = 583 | 140 (24) | 71 (51) | 172 (30) | 119 (69) | 242 (42) | 153 (63) |
| Cystic Fibrosis (CF), N = 439 | 44 (10) | 14 (32) | 57 (13) | 17 (30) | 88 (20) | 31 (35) |
| Other, N = 417 | 125 (30) | 61 (49) | 112 (27) | 65 (58) | 182 (44) | 104 (57) |
| Osteoarticular, N = 346 | 66 (19) | 38 (58) | 38 (11) | 22 (58) | 90 (26) | 57 (63) |
| Pulmonary, N = 340 | 79 (23) | 37 (47) | 66 (19) | 34 (52) | 117 (34) | 63 (54) |
| Central Nervous System (CNS), N = 264 | 57 (22) | 24 (42) | 49 (19) | 28 (57) | 88 (3) | 46 (52) |
| Skin Soft Tissue Infection (SSTI), N = 256 | 45 (18) | 17 (38) | 30 (12) | 15 (50) | 66 (26) | 31 (47) |
| Pathogen, N = 244 | 50 (21) | 24 (48) | 38 (16) | 23 (61) | 78 (32) | 45 (58) |
| Bacteremia, N = 202 | 28 (14) | 14 (50) | 26 (13) | 18 (69) | 45 (22) | 28 (62) |
| Vascular/Endocarditis, N = 168 | 45 (27) | 30 (67) | 41 (24) | 24 (59) | 72 (43) | 46 (64) |
| Upper Respiratory Infection (URI), N = 145 | 38 (26) | 22 (58) | 17 (12) | 9 (53) | 45 (31) | 26 (58) |
| Renal, N = 134 | 28 (21) | 12 (43) | 29 (22) | 16 (55) | 45 (34) | 22 (49) |
| Surgical Site, N = 88 | 17 (19) | 9 (53) | 9 (10) | 5 (56) | 24 (27) | 13 (54) |
| Unkown, N = 12 | 1 (8) | 1 (100) | 1 (8) | 1 (100) | 1 (8) | 1 (100) |
| Diagnoses limited to osteoarticular, pulmonary, SSTI, URI, renal or surgical site onlyc N = 978 | 209 (21) | 105 (50) | 149 (15) | 82 (55) | 297 (30) | 167 (56) |
| Overall (all diagnoses), N = 3433 | 776 (23) | 387 (50) | 852 (25) | 549 (64) | 1,289 (38) | 791 (61) |
Abbreviations: ED, emergency department; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; OPAT, outpatient parenteral antimicrobial therapy.
aDiagnostic categories are not mutually exclusive; OPAT episodes may be counted in more than 1 diagnostic category.
bThe following ICD-9-CM codes were selected as diagnoses related to an OPAT complication: (1) infection due to central venous catheter (99931); (2) mechanical complication of other vascular device, implant, and graft (9961); (3) other complications due to other vascular device, implant, and graft (99674); or (4) fever (78060, 78061).
cDiagnoses were mutually exclusive; only included OPAT episodes with a single diagnosis.
DISCUSSION
We examined OPAT use in a large population of pediatric Medicaid enrollees encompassing 12 states. Our results revealed 3 major findings. First, OPAT is used for children with a wide spectrum of clinical diagnoses and for the administration of a wide variety of antimicrobial agents, including both antibacterials and antifungals. Second, a substantial number of OPAT episodes included highly bioavailable antimicrobials prescribed intravenously that could potentially have been administered orally. Third, patients receiving OPAT are at high risk for requiring additional ED and inpatient hospitalizations during their OPAT episode, and the majority of these healthcare encounters were likely related to OPAT complications.
Many of our findings corroborate previously reported patterns of OPAT use in children. Osteoarticular, pulmonary, intra-abdominal, and CNS infections are well recognized as common indications for pediatric OPAT [1, 8, 16]. Ceftriaxone, cefazolin, vancomycin, clindamycin, and carbapenems were identified as commonly prescribed antimicrobials in our cohort, which is consistent with previously published data [8, 16, 17]. It is noteworthy that we identified fluconazole as the 3rd most commonly prescribed OPAT agent. Antifungal use in OPAT has not been well examined, and our data suggest that the use of antifungal therapy, including highly bioavailable azoles, is common during OPAT, especially for H/O patients. Fluconazole was also frequently prescribed among those with GI/GU, pulmonary, and CNS disease. Although determining the reasons for prescribing is beyond the scope of this work, fluconazole is often used as prophylaxis, and the need for IV administration requires evaluation given its high bioavailability.
Hematology/oncology patients accounted for the greatest number of OPAT episodes in our study. This is expected given the high risk for infections due to underlying immunosuppression; however, this group has rarely been accounted for in previous OPAT studies. Although OPAT has successfully been administered to manage febrile neutropenic episodes [18], a growing body of evidence suggest that oral antibiotics alone or oral step-down therapy after a short course of IV antimicrobials is acceptable in low-risk febrile, neutropenic patients [19–21].
The use of highly bioavailable agents for OPAT in children was relatively common in this study and warrants further scrutiny. For some patients, poor gastrointestinal absorption and concerns about tolerability or compliance with oral therapy may necessitate OPAT instead of oral administration. In pediatrics, it is unclear whether OPAT enhances adherence because drug administration is frequently parent or caregiver dependent. Although we were unable to determine the presence of comorbid conditions that might limit absorption in our study population, a substantial amount of OPAT use involved agents such as clindamycin and cefazolin for treatment of osteoarticular infections and SSTI, which are common among otherwise healthy children. The failure to switch from OPAT to oral administration when using highly bioavailable agents can result in higher medical care cost and the potential for harm without evidence of therapeutic benefit [22–24]. Conversion to peroral antimicrobials could potentially reduce OPAT-related complications because 1 in 4 antimicrobials prescribed in cases with complications were deemed highly bioavailable.
The benefits of OPAT have traditionally been viewed as outweighing the risks when used to treat infections presumably requiring IV therapy. However, recent evidence suggests that the effectiveness of oral therapy is comparable to OPAT after hospital discharge for conditions such as acute osteoarticular infections and perforated appendicitis with fewer complications due to avoidance of central catheters [22, 25–27]. Because of the high cost and potential for complications, additional studies are needed to compare the effectiveness of OPAT to oral therapy for other conditions as well as comparisons between longer and shorter durations of IV therapy.
Previous studies have highlighted that OPAT complications occur frequently, approaching 30% in some reports [11, 28]. This is consistent with our findings in which over 20% of OPAT episodes resulted in children requiring medical care in the hospital or ED setting for an OPAT complication. Outpatient parenteral antimicrobial therapy use in pediatrics may not routinely be monitored by infectious diseases specialists or stewardship programs, and recent studies indicate that a substantial percentage of pediatric OPAT is potentially avoidable [17, 29, 30]. The expansion of pediatric antimicrobial stewardship programs to encompass OPAT is a promising strategy to improve the safety and appropriateness of OPAT use. The integration of stewardship principles into clinical decision making prior to OPAT initiation is critical [31]. A proposed OPAT bundle to assure that patient selection, Infectious Diseases consultation, patient/caregiver education, and tracking of outcomes has been suggested to enhance safety practices surrounding OPAT [32]. Recognized clinical characteristics can further be used to identify those at highest risk to prevent OPAT-related hospital admissions [13].
Our study has several limitations. We used Medicaid data from 12 anonymous states, and our findings may not be generalizable to other US regions or commercially insured children. Because the database does not provide the total population of Medicaid enrollees, we were unable to determine the population-based incidence of OPAT. We used a conservative approach to define OPAT requiring both a HIT HCPCS code indicating HIT and concomitant code for antimicrobial use or retail pharmacy fill for an IV antimicrobial, and this could have resulted in an underestimation of the number of children prescribed OPAT. A HIT episode did not confirm the duration of IV antibiotic therapy administered; therefore, we were unable to make a conclusion about the impact of duration on OPAT complications. Because we were unable to review the medical charts for each OPAT episode, we may have misclassified the indication for OPAT or the reasons for subsequent healthcare use, and we were unable to determine the appropriateness of OPAT. Complication rates did vary by diagnostic category with H/O having the highest rate. When excluding diagnoses such as H/O and only evaluating diagnoses in which enteral administration of an antimicrobial is more commonly considered, the ED visit or hospitalization attributable to an OPAT complication rate remained high. Although we could have overestimated the complication rate by including fever, the majority of complications were associated with ICD-9-CM codes specific to a line complication.
CONCLUSIONS
Outpatient parenteral antimicrobial therapy frequently contains highly bioavailable antimicrobials that in some cases could be used orally, and more than 1 in 5 children treated with OPAT experience unanticipated ED visits or hospitalizations for OPAT complications. Because OPAT is prescribed for a broad range of diagnoses, pediatric specialists, including gastroenterologists, oncologists, surgeons, hospitalists, and infectious diseases physicians, must be aware of the complication risk when prescribing OPAT, especially when highly bioavailable options exist. Broadening the scope of pediatric stewardship programs to encompass hospital discharge planning for antimicrobial therapy has the potential to improve the quality and safety of OPAT use.
Supplementary Data
Supplementary materials are available at the Journal of The Pediatric Infectious Diseases Society online (http://jpids.oxfordjournals.org).
Supplementary Material
Acknowledgments
Disclaimer. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health , National Center for Advancing Translational Sciences (NCATS) , or the Agency for Healthcare Research and Quality.
Financial support. J. L. G. is supported by a Clinical and Translational Science Awards grant from NCATS awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research KL2TR000119. A. L. H. received support from grant number K08HS023320 from the Agency for Healthcare Research and Quality.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Tice AD, Rehm SJ, Dalovisio JR et al. . Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis 2004; 38:1651–72. [DOI] [PubMed] [Google Scholar]
- 2. Chapman AL, Dixon S, Andrews D et al. . Clinical efficacy and cost-effectiveness of outpatient parenteral antibiotic therapy (OPAT): a UK perspective. J Antimicrob Chemother 2009; 64:1316–24. [DOI] [PubMed] [Google Scholar]
- 3. Wai AO, Frighetto L, Marra CA et al. . Cost analysis of an adult outpatient parenteral antibiotic therapy (OPAT) programme. A Canadian teaching hospital and Ministry of Health perspective. Pharmacoeconomics 2000; 18:451–7. [DOI] [PubMed] [Google Scholar]
- 4. Barr DA, Semple L, Seaton RA. Outpatient parenteral antimicrobial therapy (OPAT) in a teaching hospital-based practice: a retrospective cohort study describing experience and evolution over 10 years. Int J Antimicrob Agents 2012; 39:407–13. [DOI] [PubMed] [Google Scholar]
- 5. Chary A, Tice AD, Martinelli LP et al. . Experience of infectious diseases consultants with outpatient parenteral antimicrobial therapy: results of an emerging infections network survey. Clin Infect Dis 2006; 43:1290–5. [DOI] [PubMed] [Google Scholar]
- 6. Wynn M, Dalovisio JR, Tice AD, Jiang X. Evaluation of the efficacy and safety of outpatient parenteral antimicrobial therapy for infections with methicillin-sensitive Staphylococcus aureus. South Med J 2005; 98:590–5. [DOI] [PubMed] [Google Scholar]
- 7. Esposito S, Noviello S, Leone S et al. . Outpatient parenteral antibiotic therapy (OPAT) in different countries: a comparison. Int J Antimicrob Agents 2004; 24:473–8. [DOI] [PubMed] [Google Scholar]
- 8. Madigan T, Banerjee R. Characteristics and outcomes of outpatient parenteral antimicrobial therapy at an academic children's hospital. Pediatr Infect Dis J 2013; 32:346–9. [DOI] [PubMed] [Google Scholar]
- 9. Olson SC, Smith S, Weissman SJ, Kronman MP. Adverse events in pediatric patients receiving long-term outpatient antimicrobials. J Pediatric Infect Dis Soc 2015; 4:119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keren R, Shah SS, Srivastava R et al. . Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr 2015; 169:120–8. [DOI] [PubMed] [Google Scholar]
- 11. Gomez M, Maraqa N, Alvarez A, Rathore M. Complications of outpatient parenteral antibiotic therapy in childhood. Pediatr Infect Dis J 2001; 20:541–3. [DOI] [PubMed] [Google Scholar]
- 12. Ruebner R, Keren R, Coffin S et al. . Complications of central venous catheters used for the treatment of acute hematogenous osteomyelitis. Pediatrics 2006; 117:1210–5. [DOI] [PubMed] [Google Scholar]
- 13. Allison GM, Muldoon EG, Kent DM et al. . Prediction model for 30-day hospital readmissions among patients discharged receiving outpatient parenteral antibiotic therapy. Clin Infect Dis 2014; 58:812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hersh AL, Lee BR, Hedican EB et al. . Linezolid use in hospitalized children. Pediatr Infect Dis J 2014; 33:e14–8. [DOI] [PubMed] [Google Scholar]
- 15. Winter ME. Basic Clinical Pharamcokinetics. Baltimore, MD; Lippincott, Williams & Wilkins; 2004. [Google Scholar]
- 16. Akar A, Singh N, Hyun DY. Appropriateness and safety of outpatient parenteral antimicrobial therapy in children: opportunities for pediatric antimicrobial stewardship. Clin Pediatr (Phila) 2014; 53:1000–3. [DOI] [PubMed] [Google Scholar]
- 17. Banerjee R, Beekmann SE, Doby EH et al. . Outpatient parenteral antimicrobial therapy practices among pediatric infectious diseases consultants: results of an emerging infections network survey. J Pediatric Infect Dis Soc 2014; 3:85–8. [DOI] [PubMed] [Google Scholar]
- 18. Wiernikowski JT, Rothney M, Dawson S, Andrew M. Evaluation of a home intravenous antibiotic program in pediatric oncology. Am J Pediatr Hematol Oncol 1991; 13:144–7. [DOI] [PubMed] [Google Scholar]
- 19. Freifeld A, Marchigiani D, Walsh T et al. . A double-blind comparison of empirical oral and intravenous antibiotic therapy for low-risk febrile patients with neutropenia during cancer chemotherapy. N Engl J Med 1999; 341:305–11. [DOI] [PubMed] [Google Scholar]
- 20. Lehrnbecher T, Phillips R, Alexander S et al. . Guideline for the management of fever and neutropenia in children with cancer and/or undergoing hematopoietic stem-cell transplantation. J Clin Oncol 2012; 30:4427–38. [DOI] [PubMed] [Google Scholar]
- 21. Vidal L, Ben Dor I, Paul M et al. . Oral versus intravenous antibiotic treatment for febrile neutropenia in cancer patients. Cochrane Database Syst Rev 2013; 10:CD003992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fraser JD, Aguayo P, Leys CM et al. . A complete course of intravenous antibiotics vs a combination of intravenous and oral antibiotics for perforated appendicitis in children: a prospective, randomized trial. J Pediatr Surg 2010; 45:1198–202. [DOI] [PubMed] [Google Scholar]
- 23. Jones M, Huttner B, Madaras-Kelly K et al. . Parenteral to oral conversion of fluoroquinolones: low-hanging fruit for antimicrobial stewardship programs? Infect Control Hosp Epidemiol 2012; 33:362–7. [DOI] [PubMed] [Google Scholar]
- 24. Kuti JL, Le TN, Nightingale CH et al. . Pharmacoeconomics of a pharmacist-managed program for automatically converting levofloxacin route from i.v. to oral. Am J Health Syst Pharm 2002; 59:2209–15. [DOI] [PubMed] [Google Scholar]
- 25. Keren R, Shah SS, Srivastava R et al. . Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr 2015; 169:120–8. [DOI] [PubMed] [Google Scholar]
- 26. Zaoutis T, Localio AR, Leckerman K et al. . Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics 2009; 123:636–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Le Saux N, Howard A, Barrowman NJ et al. . Shorter courses of parenteral antibiotic therapy do not appear to influence response rates for children with acute hematogenous osteomyelitis: a systematic review. BMC Infect Dis 2002; 2:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maraqa NF, Gomez MM, Rathore MH. Outpatient parenteral antimicrobial therapy in osteoarticular infections in children. J Pediatr Orthop 2002; 22:506–10. [PubMed] [Google Scholar]
- 29. Knackstedt ED, Stockmann C, Davis CR et al. . Outpatient parenteral antimicrobial therapy in pediatrics: an opportunity to expand antimicrobial stewardship. Infect Control Hosp Epidemiol 2015; 36:222–4. [DOI] [PubMed] [Google Scholar]
- 30. Spivak ES, Kendall B, Orlando P et al. . Evaluation of outpatient parenteral antimicrobial therapy at a Veterans Affairs hospital. Infect Control Hosp Epidemiol 2015; 36:1103–5. [DOI] [PubMed] [Google Scholar]
- 31. Patel S, Abrahamson E, Goldring S et al. . Good practice recommendations for paediatric outpatient parenteral antibiotic therapy (p-OPAT) in the UK: a consensus statement. J Antimicrob Chemother 2015; 70:360–73. [DOI] [PubMed] [Google Scholar]
- 32. Muldoon EG, Snydman DR, Penland EC, Allison GM. Are we ready for an outpatient parenteral antimicrobial therapy bundle? A critical appraisal of the evidence. Clin Infect Dis 2013; 57:419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.