Abstract
Background
Human immunodeficiency virus (HIV)-infected children are particularly susceptible to acute respiratory infections (ARIs). We determined incidence and cofactors for ARIs in HIV-infected infants receiving antiretroviral therapy (ART).
Methods
Human immunodeficiency virus-infected infants initiated ART at ≤12 months of age and were observed monthly for 2 years in Nairobi. Acute respiratory infection rates and cofactors were determined using Andersen-Gill models, allowing for multiple events per infant.
Results
Among 111 HIV-infected infants, median age at ART initiation was 4.5 months. Pre-ART median CD4% was 19%, and 29% had wasting. During 24-months follow-up while on ART, upper respiratory infection (URI) and pneumonia rates were 122.6 and 34.7 per 100 person-years (py), respectively. Infants with higher pre-ART viral load (VL) (plasma HIV ribonucleic acid [RNA] ≥7 log10 copies/mL) had 4.12-fold increased risk of pneumonia (95% confidence interval [CI], 2.17–7.80), and infants with wasting (weight-for-height z-score < −2) had 2.87-fold increased risk (95% CI, 1.56–5.28). Infants with both high pre-ART VL and wasting had a higher pneumonia rate (166.8 per 100 py) than those with only 1 of these risk factors (44.4 per 100 py) or neither (17.0 per 100 py). Infants with exposure to wood fuel had significantly higher risk of URI (hazard ratio [HR] = 1.82; 95% CI, 1.44–2.28) and pneumonia (HR = 3.31; 95% CI, 1.76–6.21).
Conclusions
In early ART-treated HIV-infected infants, higher HIV RNA and wasting before ART were independent risk factors for pneumonia. Wood fuel use was associated with URI and pneumonia. Additional data on air pollution and respiratory outcomes in HIV-infected children may help optimize interventions to improve their lung health.
Keywords: acute respiratory infections, HIV, home air pollution, infants, pneumonia
Globally, acute respiratory tract infections (ARIs) are a leading cause of under-5 child morbidity and mortality in developing countries [1–3]. In resource-poor settings, low birth weight, lack of exclusive breastfeeding, malnutrition, crowding, poor vaccine coverage, and indoor air pollution are each risk factors for ARIs and ARI-related mortality [2, 4, 5].
Most pediatric human immunodeficiency virus (HIV) and childhood pneumonia-related death is concentrated in Africa [3]. Before widespread antiretroviral treatment (ART), untreated HIV-infected children had 23.5 times higher hospitalizations for acute lower respiratory tract infections (ALRIs) compared with HIV-unexposed children [6]. A 2010 meta-analysis estimated a 6.5-fold higher risk of hospitalization for pneumonia and a 6-fold increased risk of mortality from pneumonia in HIV-infected children, who were often untreated [4].
In pediatric cohorts on ART, the ARI burden has persisted [7–10], with some evidence of decline. The pneumonia rate decreased from 50.6 to 18.1 per 100 person-years (py) after ART in Cote d'Ivoire [11]. The landmark Children with HIV Early Antiretroviral Therapy (CHER) trial demonstrated 76% reduced mortality in South African infants randomized to early (by age 6–12 weeks) versus deferred ART, and infants randomized to early ART also had lower severe pneumonia (12.2 vs 26.5 per 100 py) [12]. At the same time, a number of studies have implicated pneumonia in mortality and hospitalization in children on ART [12–15].
Older HIV-infected children and adolescents with either poor immune status before ART or poor response to ART have higher risk of ARIs [10, 16], and we hypothesize that a subset of treated infants may continue to have high risk of pneumonia, in spite of ART. Like HIV, indoor air pollution may compromise immune responses or contribute to inflammation [17], and the combined burden of exposure to indoor air pollution and severe HIV disease may be particularly harmful.
We examined the on-ART (“post-ART”) incidence rates and cofactors for ARIs and pneumonia among HIV-infected infants receiving early ART. We hypothesized that infants with high viremia and poor immune status before ART would have higher ARIs. We also explored the relationship between indoor air pollution and risk of ARIs in this group.
METHODS
Study Populations and Follow-Up
Study participants were from Optimizing Pediatric HIV-1 Therapy (OPH) 03 (NCT00428116) and OPH 612 (NCT00427297) cohorts (2007–2010), both based in Nairobi Kenya. Infants were identified at prevention of mother-to-child transmission (PMTCT) clinics or in hospital wards as described previously [14, 18]. Both studies included only infants who initiated ART at <12 months of age. Infants were eligible for OPH612 if they had a prior nevirapine (NVP) exposure with no detectable resistance to nonnucleoside reverse-transcriptase inhibitors, based on an in-house population-based resistance test performed as described previously [19]. In brief, viral ribonucleic acid (RNA) was extracted from 140 µL plasma using a QIAmp viral RNA kit (QIAGEN, Valencia, California), amplified by reverse-transcription polymerase chain reaction, and sequenced on an ABI Prism 3100 in Nairobi. A consensus sequence was submitted to the Stanford University HIV Drug Resistance Database (http://hivdb.stanford.edu/) for interpretation of drug resistance profiles. Before 2009, infants with suspected active tuberculosis were excluded, and thereafter they were included. Infants in OPH03 were observed for a 2-year prerandomization phase on ART. Infants enrolled in OPH612 were randomized to either lopinavir-boosted ritonavir (LPV/r) or NVP-based ART regimen and followed thereafter. Studies were approved by Kenyatta National Hospital/University of Nairobi Ethical Review Board and University of Washington Institutional Review Board.
In accordance with World Health Organization (WHO) and Kenyan guidelines, infants received trimethoprim-sulfamethoxazole (co-trimoxazole) prophylaxis and received bacille Calmette-Guerin (BCG) at birth, pentavalent (for diphtheria, pertussis, tetanus, hepatitis B, and Haemophilus influenza type b) at 6, 10, and 14 weeks, and polio vaccinations at birth, 6, 10, and 14 weeks.
Data Collection
After enrollment, infants received a physical examination, and infants and biological mothers provided blood specimens. Caregivers provided sociodemographics. Infants attended monthly follow-up visits, at which time current respiratory illness and symptoms were ascertained through questionnaires and a physical exam. Infants also came in for unscheduled visits when ill, and clinical assessment and treatment were given by study clinicians. This analysis includes follow-up data for up to 2 years postenrollment, or until attrition.
Blood samples were ascertained for plasma HIV RNA using the Gen-Probe HIV Viral Load Assay [20], and CD4% and count levels were determined using flow cytometry. Among children still in follow-up in 2013 (4–6 years post-ART initiation), the type of fuel used in the household was determined (dung, firewood, charcoal, paraffin [kerosene], or liquid petroleum gas [LPG]). Usage of more than 1 fuel could be reported.
Respiratory Outcomes
Respiratory conditions (ARIs) assessed at enrollment and during monthly clinic visits included upper respiratory infection (URI), otitis media, bronchiolitis, pneumonia, and pulmonary tuberculosis (tuberculosis). Diagnoses were made by clinicians. The case definition of URI and pneumonia were based on WHO definitions [21] and were similar to definitions used in prior studies [22]. The case definition of URI was a history of cough or runny nose with or without fever, with absent fast breathing (defined as <60 breaths/minute [0–2 months], <50 breaths/minute [2–12 months], and <40 breaths/minute [12–59 months]) and normal chest exam. The pneumonia case definition was presentation with cough and fast breathing, with possibility of respiratory examination consistent with tachypnea, lower chest in-drawing, nasal flaring, grunting, or coarse crackles on auscultation. Clinicians used the Keith Edwards scoring system to diagnose tuberculosis [23], as per Kenyan Ministry of Health guidelines.
Statistical Analysis
Z-scores for weight-for-age (WAZ), height-for-age (HAZ), weight-for-height (WHZ), and head circumference-for-age (HCZ) were calculated using the 2006 WHO reference population [24]. Incidences of respiratory infections post-ART initiation were calculated, based on diagnoses made by clinicians at follow-up study visits, and excluding conditions present by ART initiation. The association between ARI and cofactors for ARI were assessed with Cox regression models using the Andersen-Gill method to allow for multiple events per infant over the study period. Primary cofactors of interest were pre-ART plasma HIV RNA and CD4% and home fuel exposure (using dichotomous indicators for any wood, charcoal, kerosene, or use of LPG, a low polluting fuel type). High pre-ART viremia was defined as plasma HIV RNA ≥7 log10 copies/mL, and definitions for underweight, stunting, wasting, and microcephaly were WAZ, HAZ, WHZ, and HCZ < −2, respectively. Multivariate analyses were performed to determine independent cofactors for respiratory tract conditions. Incidence rates were calculated for months 0–3, 3–6, 6–12, and 12–24 during ART. Indicators for socioeconomic status were not included in multivariate analyses restricted to the 7 to 24-month timeframe due to co-linearity. Dichotomous indicators for fuel represented presence or absence of each fuel type, allowing for multiple fuel exposures. To further examine pneumonia, Kaplan–Meier survival methods were used to estimate time to first event, with ART initiation as the time origin. Cox proportional hazards regression was used to evaluate cofactors.
Secondary cofactors of interest included low birth weight, infant feeding practices, receipt of PMTCT (OPH03 only) nutritional status, prior hospitalization, advanced WHO clinical stage, and socioeconomic indicators. Cofactors for any LPG use were assessed using χ2 or Fisher's exact tests (for dichotomous variables) and Wilcoxon rank–sum tests (for continuous variables). Stata 11 SE (StataCorp, College Station, Texas) was used for statistical analysis.
RESULTS
Study Population
Among 157 enrolled infants, 23 had initiated ART before enrollment, and 23 infants did not initiate ART (15 infants died, 3 infants were lost to follow-up, 4 infants withdrew, and 1 infant was exited due to early closure of the OPH612 study). One-hundred eleven infants initiated ART after enrollment at a median age of 4.5 months. Infants had a median CD4% of 19%, a median CD4 count of 1282 cells/μL, and a median plasma HIV-1 RNA of 6.6 log10 copies/mL (Table 1). Nearly half (46.9%) had a WHO clinical stage 3 or 4 diagnosis, and among these 52 infants, 64% had severe recurrent bacterial pneumonia. Other common diagnoses were unexplained moderate or severe malnutrition (17%) and unexplained persistent diarrhea (15%). Each of these diagnoses often occurred in combination with other diagnoses.
Table 1.
Summary of Baseline Characteristics of Infants Who Initiated ART
| Characteristic | N = 111 | Median (IQR) or N (%) |
|---|---|---|
| Infant Birth and Pre-ART Clinical Characteristics | ||
| Male | 111 | 54 (48.7) |
| Birth weight (kg) | 107 | 3 (2.7–3.4) |
| Birth weight (<2.5 kg) | 107 | 13 (12.1) |
| Ever breastfed | 95 | 85 (89.5) |
| PMTCT received by mother or infant | 107 | 72 (67.3) |
| HIV diagnosis in hospital | 111 | 70 (63.1) |
| Age at ART initiation (months) | 111 | 4.5 (3.7–6.9) |
| CD4% | 111 | 19 (14–25) |
| CD4% <15 | 111 | 31 (27.9) |
| CD4+ T-cell count (cells/μL) | 111 | 1282 (750–1939) |
| Plasma HIV-1 RNA (log10 copies/mL) | 105 | 6.6 (6.0–7.0) |
| WHO Stage 3 or 4 | 111 | 52 (46.9) |
| Prior hospitalization | 111 | 65 (58.6) |
| Pre-ART Growth Status | ||
| WAZ | 111 | −2.3 (−3.8 to −1.0) |
| Underweight (WAZ < −2) | 63 (56.8) | |
| HAZ | 111 | −2.0 (−3.1 to −0.9) |
| Stunted (HAZ < −2) | 56 (50.5) | |
| WHZ | 111 | −0.8 (−2.3 to 0.2) |
| Wasting (WHZ < −2) | 32 (28.9) | |
| HCZ | 111 | −0.9 (−1.7 to 0.4) |
| Microcephalic (HCZ < −2) | 18 (16.2) | |
| Socioeconomic Indicators | ||
| Caregiver employed | 110 | 75 (68.2) |
| Household monthly rent (KES) | 100 | 1500 (1200–2500) |
| Caregiver education (years) | 96 | 8.5 (8–11) |
| Social History of Caregiver | ||
| Age (years) | 110 | 26 (23–31) |
| Biological mother | 111 | 108 (97.3) |
| Married | 111 | 87 (78.4) |
| Home | ||
| One-room house | 111 | 86 (77.5) |
| Number of people in house | 111 | 4 (3–5) |
| Number of people per room | 111 | 3.5 (3–5) |
Abbreviations: ART, antiretroviral therapy; HAZ, height-for-age Z-score; HCZ, head circumference Z-score; HIV, human immunodeficiency virus; IQR, interquartile range; KES, Kenyan Shillings; PMTCT, prevention of mother-to-child transmission; RNA, ribonucleic acid; WAZ, weight-for-age Z-score; WHO, World Health Organization; WHZ, weight-for-height Z-score.
Most infants (89.5%) had breastfed, and 67.3% had received or had mothers who received PMTCT. Many infants (28.9%) had wasting (WHZ < −2). Nearly all infants had completed their expected immunizations by enrollment or shortly thereafter for BCG (110; 99%), polio (106; 96%), and pentavalent (106; 96%). Likewise, 110 (99%) infants had started co-trimoxazole prophylaxis before enrollment. Most caregivers (97.3%) were biological mothers. Most (77.5%) lived in a 1-room house.
Respiratory Infection Burden
Among 111 infants included in ART analysis, 103 provided post-ART morbidity data. The numbers remaining in follow-up at 6, 12, and 24 months were 78, 75, and 71, respectively. Among 81 survivors, the median follow-up time was 22.8 months (interquartile range [IQR], 22.6–23.2), and among 22 infants who died, median time to death was 2.3 months (IQR, 1.6–4.1). Seven infants were lost to follow-up at a median of 6.2 (IQR, 2.7–12.8) months.
During 2 years of follow-up on ART, 78 (75.7%) infants had at least 1 episode of URI, and 50 (48.5%) infants had recurrent episodes. Thirty-six infants (35.0%) had at least 1 pneumonia, and 9 (8.7%) infants had recurrent episodes. Three (3%) infants had an episode of tuberculosis.
The overall URI incidence rate was 122.6 per 100 py, and the overall pneumonia incidence rate was 34.7 per 100 py (Table 2). Rates for otitis media, bronchiolitis, and tuberculosis were 15.7 per 100 py, 5.4 per 100 py, and 2.0 per 100 py, respectively. For each condition, rates were highest during the first 3 months on ART (Table 2). For example, pneumonia declined from 54.7 per 100 py during months 0–3 to 34.9 per 100 py during months 3–6.
Table 2.
Incidence of Respiratory Conditions Among HIV-Infected Infants by Duration of ART, 24 Months ART, and Interim ART Periods 0–3, 3–6, 6–12, and 12–24 Months
| Respiratory Conditions and Duration on ART | Number of Respiratory Events | Incidence/100 Person-Yearsa (95% CI) |
|---|---|---|
| URI | ||
| Overall | 180 | 122.6 (105.9–141.9) |
| 0–3 months | 36 | 151.4 (109.2–210.0) |
| 3–6 months | 28 | 139.5 (96.4–202.0) |
| 6–12 months | 42 | 111.7 (82.6–151.2) |
| 12–24 months | 74 | 113.2 (90.1–142.2) |
| Otitis media | ||
| Overall | 23 | 15.7 (10.4–23.6) |
| 0–3 months | 5 | 21.0 (8.8–50.5) |
| 3–6 months | 3 | 14.9 (4.8–46.3) |
| 6–12 months | 7 | 18.6 (8.9–39.1) |
| 12–24 months | 8 | 12.2 (6.1–24.5) |
| Bronchiolitis | ||
| Overall | 8 | 5.4 (2.7–10.9) |
| 0–3 months | 3 | 12.6 (4.1–39.1) |
| 3–6 months | 1 | 5.0 (.7–35.4) |
| 6–12 months | 3 | 8.0 (2.6–24.7) |
| 12–24 months | 1 | 1.5 (.2–10.9) |
| Pneumoniab | ||
| Overall | 51 | 34.7 (26.4–45.7) |
| 0–3 months | 13 | 54.7 (31.8–94.2) |
| 3–6 months | 7 | 34.9 (16.6–73.1) |
| 6–12 months | 15 | 39.9 (24.1–66.2) |
| 12–24 months | 16 | 24.5 (15.0–40.0) |
| Tuberculosisc | ||
| Overall | 3 | 2.0 (.7–6.3) |
| 0–3 months | 2 | 8.4 (2.1–33.6) |
| 3–6 months | — | — |
| 6–12 months | 1 | 2.7 (.4–18.9) |
| 12–24 months | — | — |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; URI, upper respiratory infection.
a Total person-years = 146.8.
b Pneumonia episodes include 1 episode that preceded a tuberculosis diagnosis.
c Tuberculosis episodes include 2 episodes with pulmonary tuberculosis diagnoses and 1 episode with concurrent pulmonary and extrapulmonary tuberculosis diagnoses.
Cofactors for Upper Respiratory Infection and Pneumonia During 2 Years Follow-Up on Antiretroviral Therapy
In analyses allowing for multiple events per infant, infants with high pre-ART plasma HIV RNA level had a higher risk of pneumonia (hazard ratio [HR] = 2.00; 95% confidence interval [CI], 1.20–3.34 per log10 copies/mL increase; P = .008) (Table 3), and those with high plasma viremia (HIV RNA ≥7 log10 copies/mL) had a 4-fold increased risk of pneumonia (HR = 4.12; 95% CI, 2.17–7.80; P < .001). Infants who had wasting before ART had a nearly 3-fold increased risk of pneumonia (HR = 2.87; 95% CI, 1.56–5.28; P = .001). Indicators of lower socioeconomic status, including lower monthly rent, and fewer years of caregiver education were each associated with higher risk of pneumonia. Results were similar in multivariate analyses adjusted for caregiver years of education (high viremia [HIV RNA ≥7 log10 copies/mL], adjusted HR [aHR] = 3.02, 95% CI, 1.58–5.78; P = .001; wasting, aHR = 3.14, 95% CI, 1.69–5.81; P < .001). In a multivariate model restricted to months 7-24, high pre-ART viremia and wasting were also associated with higher risk of pneumonia (high viremia, aHR = 2.90, 95% CI, 1.60–5.27; P < .001), wasting (aHR = 3.55, 95% CI, 1.91–6.57; P < .001). Lack of viral suppression (HIV RNA ≥3 log10 copies/mL, aHR = 3.01, 95% CI, 1.32–6.85; P = .009) and wasting (aHR, 1.93, 95% CI, 1.00–3.70; P = .05) at 6 months were each associated with higher pneumonia during months 7–24. In contrast to viremia, neither pre-ART nor 6-month CD4% were associated with pneumonia. Neither pre-ART growth, CD4%, nor plasma virus level were associated with URI incidence.
Table 3.
Univariate Analyses of Cofactors for URI and Pneumonia Incidence in Early-Treated HIV-Infected Infants
| URIa | Pneumoniaa | |||
|---|---|---|---|---|
| Potential Cofactor | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value |
| Infant Birth and Pre-ART Clinical Characteristics | ||||
| Male | 1.08 (.81–1.45) | .6 | 0.72 (.37–1.40) | .3 |
| Birth weight (per kg increase)b | 1.0 (.74–1.38) | 1.0 | 1.46 (.77–2.77) | .3 |
| Ever breastfedc | 1.02 (.69–1.51) | .9 | 0.93 (.34–2.53) | .9 |
| Received PMTCTd | 1.29 (.92–1.80) | .1 | 1.07 (.53–2.16) | .9 |
| ART initiation age (per month in age) | 0.96 (.91–1.02) | .2 | 1.16 (1.03–1.31) | .02 |
| CD4% (per percentage increase) | 1.01 (.99–1.03) | .4 | 0.98 (.94–1.01) | .2 |
| Plasma HIV RNA (per log10 c/mL increase)e | 0.94 (.79–1.12) | .5 | 2.00 (1.20–3.34) | .008 |
| Plasma HIV RNA ≥7 log10 c/mL | 0.96 (.67–1.36) | .8 | 4.12 (2.17–7.80)f | <.001 |
| WHO Stage 3 or 4 | 0.98 (.72–1.33) | .9 | 1.92 (1.01–3.68) | .05 |
| Pre-ART Growth Status | ||||
| Underweight (WAZ < −2)g | 0.87 (.65–1.17) | .4 | 1.45 (.76–2.77) | .3 |
| Stunted (HAZ < −2) | 0.81 (.61–1.09) | .2 | 1.02 (.53–1.96) | 1.0 |
| Wasting (WHZ < −2) | 0.95 (.65–1.38) | .8 | 2.87 (1.56–5.28)f | .001 |
| Microcephalic (HCZ < −2) | 0.93 (.54–1.63) | .8 | 0.56 (.22–1.45) | .2 |
| Socioeconomic Indicators, Social History, and Home Characteristics | ||||
| Caregiver education (per year increase)h | 0.97 (.92–1.02) | .2 | 0.85 (.75–0.96) | .007 |
| Household rent (per 1000 KES increase)i | 0.95 (.89–1.01) | .09 | 0.61 (.36–1.05) | .08 |
| One-room house | 1.04 (.75–1.46) | .8 | 1.41 (.65–3.05) | .4 |
| People per room | 0.99 (.90–1.08) | .7 | 1.15 (.90–1.47) | .3 |
Abbreviations: aHR, adjusted hazard ratio; ART, antiretroviral therapy; c, copies; CI, confidence interval; HAZ, height-for-age Z-score; HCZ, head circumference Z-score; HIV, human immunodeficiency virus; HR, hazard ratio; KES, Kenyan Shillings; OPH, Optimizing Pediatric HIV-1 Therapy; PMTCT, prevention of mother-to-child transmission; RNA, ribonucleic acid; URI, upper respiratory infection; WAZ, weight-for-age Z-score; WHO, World Health Organization; WHZ, weight-for-height Z-score.
aNumber of infants = 103; total person-years = 146.8; no. URI events = 180; no. pneumonia events = 51, except as noted.
bNumber of infants = 99; total person-years = 139.6; no. URI events = 169; no. pneumonia events = 50.
cNumber of infants = 91; total person-years = 139.5; no. URI events = 171; no. pneumonia events = 47.
dAnalyses restricted to infants in the OPH 03 cohort; no. infants = 70; total person-years = 102.1; no. URI events = 137; no. pneumonia events = 37.
eNumber of infants = 97; total person-years = 139.0; no. URI events = 166; no. pneumonia events = 47.
fIn multivariate analyses, adjusted for caregiver years of education, HIV RNA ≥7 log10 copies/mL (aHR = 3.02; 95% CI, 1.58–5.78; P = .001), wasting (aHR = 3.14; 95% CI, 1.69–5.81; P < .001).
gPneumonia HR = 0.83 (95% CI, .69–.99) (P = .04) per unit increase in WAZ.
hNumber of infants = 89; total person-years = 126.8; no. URI events = 160; no. pneumonia events = 47.
iNumber of infants = 93; total person-years = 127.9; no. URI events = 160; no. pneumonia events = 44.
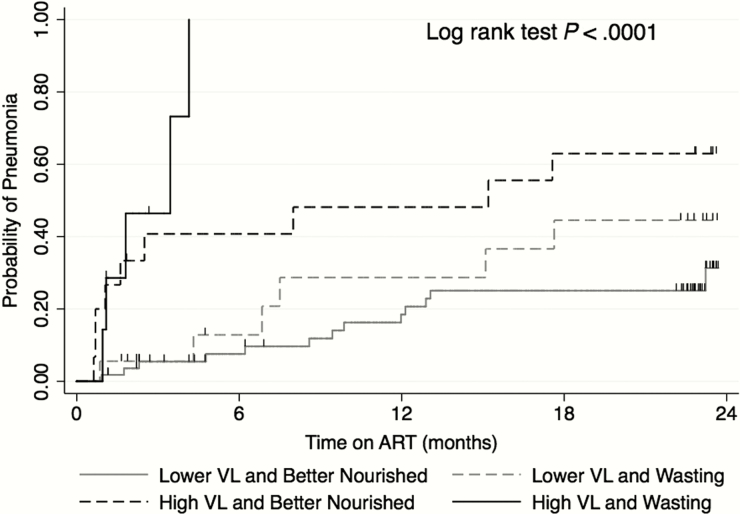
Infants with combined high pre-ART viremia and wasting had an extraordinarily high rate of pneumonia (166.8 episodes per 100 py; 95% CI, 96.9–287.3), representing a 10-fold increase (aHR = 9.48; 95% CI, 5.39–16.67; P < .001) in a model adjusted for caregiver education. The rate of pneumonia was lower in infants with either of these risk factors alone (44.4 per 100 py; 95% CI, 28.3–69.6), corresponding to a 3-fold increased risk in each case (high viremia and better nourished, aHR = 3.00, 95% CI, 1.23–7.30, P = .02; and lower viremia with wasting, aHR = 3.10, 95% CI, 1.22–7.90; P = .02). The rate was lower still in infants with neither risk factor (17.0 per 100 py; 95% CI, 10.2–28.2). Results were similar in survival analyses that allowed for 1 event per person (Figure 1).
Figure 1.
Kaplan–Meier curve depicting probability of pneumonia in early-treated human immunodeficiency virus (HIV)-infected infants by pre- antiretroviral therapy (ART) virological and nutritional status. In Cox proportional hazards models examining time to first event and adjusted for caregiver education, infants with high viremia with wasting had adjusted hazard ratio (aHR) = 8.48, 95% confidence interval (CI), 3.84–18.75; P < .001; high viremia and better nourished had aHR = 3.61, 95% CI, 1.30–9.99; P = .01; and lower viremia with wasting had aHR = 2.71, 95% CI, 1.12–6.57; P = .03, compared with those with lower viremia and better nourishment. Abbreviations: Better Nourished, WHZ ≥ −2; High VL, plasma HIV RNA ≥7 log10 copies/mL; Lower VL, plasma HIV RNA <7 log10 copies/mL; RNA, ribonucleic acid; VL, viral load; Wasting, WHZ < −2; WHZ, weightfor-height Z-score.
Prevalence of Fuel Usage and Relation Between Fuel Use and Risk of Acute Respiratory Infection
Sixty-two children had data for household fuel use (46 who initiated ART at enrollment, and 16 who initiated before enrollment), a proxy for exposure to indoor air pollution. Four (6.5%) children lived in households with firewood or both firewood and dung exposure (both high polluting), 46 (74.2%) had charcoal exposure, 45 (72.6%) had kerosene exposure (both medium polluting), and 5 infants (8.1%) had neither charcoal nor kerosene. Fifteen (24.2%) infants lived in households with use of LPG (low polluting), which was generally used in combination with high or medium polluting fuels. Cofactors for use of any LPG included male infant (80% vs 46.8%; P = .04), higher education level (median = 12, IQR = 10–13 vs median = 10, IQR = 8–12; P = .02), living in a home with >1 room (66.7% vs 28.3%; P = .01), and HIV diagnosis in hospital (100% vs 52.6%; P = .02). Infants with LPG use had a lower CD4% before ART (median = 16%, IQR = 12%–21% vs median = 19%, IQR = 14%–25%; P = .05).
Forty-six infants had fuel exposure data, with 2 having exposure to wood and 44 infants without wood and with exposure to charcoal alone (N = 6), kerosene alone (N = 7), LPG alone (N = 2), or a combination of fuels (N = 29). Compared to other infants, infants with wood fuel exposure had significantly increased risk of URI (HR = 1.82; 95% CI, 1.44–2.28; P < .001) (Table 4) and pneumonia (HR = 3.31; 95% CI, 1.76–6.21; P < .001). Results were similar after adjusting for pre-ART HIV RNA level and WHZ (URI, aHR = 2.04, 95% CI, 1.55–2.67; P < .001 and pneumonia, aHR = 3.97, 95% CI, 1.83–8.58; P < .001). In adjusted models examining intervals of time during ART, the relation between wood fuel exposure and URI was only significant during months 7–24 (aHR = 2.31; 95% CI, 1.69–3.16; P < .001). There were no pneumonia episodes during months 0–6 among infants exposed to wood fuel; during months 7–24, these infants had a 5-fold increased risk of pneumonia (aHR = 5.35; 95% CI, 1.89–15.18; P = .002).
Table 4.
Univariate Models for Fuel Use as Cofactors for URI and Pneumonia Incidence in Early-Treated HIV-Infected Infants
| URI | Pneumonia | ||||||
|---|---|---|---|---|---|---|---|
| N | Events/Person-Time (Years) | Incidence/100 py (95% CI) | HR (95%CI) | Events/Person-Time (Years) | Incidence/100 py (95% CI) | HR (95% CI) | |
| Wood | |||||||
| Yes | 2 | 8/3.8 | 210.4 (105.2–420.7) | 1.82 (1.44–2.28)a | 2/3.8 | 52.6 (13.2–210.3) | 3.31 (1.76–6.21)b |
| No | 43 | 100/82.1 | 121.8 (100.1–148.1) | 13/82.1 | 15.8 (9.2–27.3) | ||
| Charcoal | |||||||
| Yes | 10 | 86/68.9 | 124.9 (101.1–154.3) | 0.87 (.46–1.65) | 11/68.9 | 16.0 (8.8–28.4) | 0.76 (.27–2.18) |
| No | 36 | 26/19.0 | 137.0 (93.3–201.2) | 4/19.0 | 21.1 (7.9–56.2) | ||
| Kerosene | |||||||
| Yes | 10 | 88/68.7 | 128.2 (104.0–158.0) | 1.10 (.66–1.84) | 10/68.7 | 14.6 (7.8–27.1) | 0.56 (.15–2.04) |
| No | 36 | 24/19.2 | 125.2 (83.9–186.8) | 5/19.2 | 26.1 (10.9–62.7) | ||
| Gas/LPG | |||||||
| Yes | 8 | 12/15.2 | 79.0 (44.9–139.2) | 0.58 (.23–1.45) | 2/15.2 | 13.2 (3.3–52.7) | 0.56 (.10–5.10) |
| No | 38 | 100/72.6 | 137.6 (113.1–167.5) | 13/72.6 | 17.9 (10.4–30.8) | ||
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio; LPG, liquid petroleum gas; py, person-years; URI, upper respiratory infection.
a P < .001.
b P < .001.
DISCUSSION
In this study of ARIs in HIV-infected infants in Kenya, the 2-year incidence rate for pneumonia was 34.7 per 100 py, and the incidence rate for URI was 122.6 per 100 py. Key cofactors associated with risk of pneumonia were pre-ART viremia and wasting. Infants with combined high plasma viremia and wasting had extremely high incidence of pneumonia—166.8 per 100 py. Infants with either high viremia or wasting had lower risk (44.4 per 100 py), whereas infants with neither risk factor had the lowest incidence of pneumonia (17.0 per 100 py). The moderate risk group had pneumonia incidence comparable to infants in the CHER cohort (44.3 per 100 py) who initiated ART at age 12 weeks and were not immunocompromised before ART [12]. Importantly, infants were enrolled before Kenyan rollout of pneumococcal conjugate vaccine. Rates of pneumonia in early-treated HIV-infected infants with access to this vaccine may be lower.
The overall pneumonia incidence in this study was 34.7 per 100 py over 24 months. Estimates for Kenya are similar: the WHO estimates that incidence of childhood (0−5 years) pneumonia in Kenya is 31–40 per 100 py [1]. The tuberculosis rate in this study was 2.0 per 100 py, substantially higher than estimates for the general population (167 cases of pediatric [0–14 years] tuberculosis per 100 000 in Kenya) [25]. Data for URI incidence in the general population in this region are scant; a cross-sectional study in rural Uganda found a prevalence of 37% among children age <2 years [26].
In this study, rates for upper and lower respiratory illnesses were highest during the first 3 months on ART. We previously observed that in older HIV-infected children initiating ART, pneumonia and tuberculosis were the most common morbidities [27], with the most severe morbidity occurring during first few months of ART, and likely before full immune reconstitution [27].
We found that infants with high viremia had a 4-fold increased risk of pneumonia. Peak plasma virus levels are associated with HIV disease progression and mortality in children [28–34]. Viremia before ART may be a key marker for risk of pneumonia in infants, who rapidly progress to severe HIV disease [30]. Prior studies have reported associations with immunosuppression and increased risk of pneumonia and opportunistic infections [10, 16]. However, we found no significant association between pre-ART CD4% and pneumonia risk.
One-third of infants had wasting, which was associated with significantly higher pneumonia. Undernutrition is associated with ARIs in hospital- and community-based studies [35, 36]. Undernutrition is also associated with mortality in HIV-infected infants in this cohort and others [14, 37, 38]. Undernutrition may worsen immunosuppression or impede immune reconstitution. Malnourished children also have vitamin and mineral deficiencies, which contribute to poor immune function; reduced cell-mediated immunity is associated with higher risk of childhood ARIs [35].
We found that lower caregiver education and household rent were each associated with a higher risk of pneumonia. In HIV-uninfected children, poor maternal education and poverty have each been associated with increased risk of ALRI mortality [5]. Poverty is linked to household crowding, which is itself a commonly cited risk factor for ARIs [39–41]. The majority of infants in this cohort lived in a 1-room home, making it difficult to evaluate crowding as a risk factor in this study.
The majority of households (92%) in this periurban cohort used charcoal or kerosene for cooking, and fewer used wood fuel (6%). Twenty-four percent of households used at least some LPG for cooking. These proportions are consistent with recent Kenyan demographic survey data for urban households [42]. Use of LPG, a more expensive type of fuel than kerosene, charcoal or wood, was associated with living in a house with >1 room. Infants with lower pre-ART CD4% and infants who had their HIV diagnosis in hospital were each more likely to live in households with LPG, perhaps because women with resources sought care at private clinics, which did not offer routine antenatal or infant HIV testing.
Wood fuel is associated with higher risk of respiratory illness than either charcoal or kerosene [17]. We observed significantly higher rates of URI and pneumonia among infants with wood exposure, in spite of small numbers. This study was limited to self-reported household fuel as a proxy for indoor air pollution exposure, and fuel usage was not quantified. Additional studies are needed to explore interactions between HIV, air pollution, and ALRI risk in children.
A strength of this study was the intensive monthly assessment of ARI incidence. To our knowledge, only 1 other study has reported ARI rates in empirically treated infants [12]. Previous studies involved older children [9, 11] or infants not provided with early ART [8, 10, 15]. Infants in our study were severely ill at enrollment, with severe malnutrition and immune compromise, both common in HIV-infected infants in Africa [37]. To date, no studies have examined the combined effects of HIV and household air pollution on respiratory infection risk in infants.
Nevertheless, this study was a secondary analysis, and it lacked a standardized protocol to diagnose respiratory infections, which may have led to missed or incorrect diagnoses. This study did not distinguish between severe and mild pneumonia. Many infants died before ART, limiting our ability to identify infant characteristics associated with higher ARI rates. Results presented here may not be generalizable to infants with access to the pneumococcal conjugate vaccine. Ascertainment of home air pollution exposure occurred 4–6 years after ascertainment of infant ARI outcomes.
CONCLUSIONS
In summary, ARI rates in early-treated HIV-infected infants may continue to be high during early months of ART. Infants with high viremia, wasting, or both are at particularly high risk, and careful monitoring of infants with these risk factors is warranted. The contribution of household air pollution to risk of ARI in HIV-infected children merits further study. Combined interventions such as nutritional support, early initiation of ART, and interventions to reduce home air pollution may optimize long-term lung health in these children.
Notes
Acknowledgments. We thank Dr. Lisa Cranmer, the Kenya Research and Training Center, the Kizazi working group, and the UW Global Center for Integrated Health of Women, Adolescents and Children for support and insightful discussions during the preparation of this article. We also thank the Optimizing Pediatric HIV-1 Therapy (OPH) administrative, clinic, and data management staff in Nairobi, Kenya, and in Seattle, Washington for their ongoing support, commitment, and participation. We are most grateful to the OPH Study participants, without whom this research would not be possible.
Financial support. The Optimizing HIV-1 Therapy Study was supported by the National Institute of Child Health and Human Development Grant 2 R01 HD023412. Field site and biostatistical support were provided by the University of Washington International and Biometrics Cores of the Center for AIDS Research, a National Institutes of Health (NIH)-funded program (P30 AI027757), which is supported by the following NIH Institutes and Centers: National Institute of Allergy and Infectious Diseases; National Cancer Institute; National Institute of Mental Health; National Institute on Drug Abuse; Eunice Kennedy Shriver National Institute of Child Health and Human Development; National Heart, Lung, and Blood Institute; and National Center for Complementary and Alternative Medicine. S. B.-N. was supported by a New Investigator Award supported by the Developmental Core of Center for AIDS Research (P30 AI027757), 2 R01 HD023412, and the National Institute of Neurological Disorders and Stroke (NINDS) Grant 5K01NS080637. D. W. was supported by the Global Research Initiative Program, Social Science (R01 TW007632). G. J.-S. was supported by NIH Grant K24 HD054314. C. J. K. was supported by the UW Center for Ecogenetics and Environmental Health, a National Institute of Environmental Health Sciences-funded program (5P30ES007033-19).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Rudan I, Boschi-Pinto C, Biloglav Z et al. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ 2008; 86 : 408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rudan I, O'Brien KL, Nair H et al. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health 2013; 3 : 010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu L, Johnson HL, Cousens S et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012; 379 : 2151–61. [DOI] [PubMed] [Google Scholar]
- 4. Theodoratou E, McAllister DA, Reed C et al. Global, regional, and national estimates of pneumonia burden in HIV-infected children in 2010: a meta-analysis and modelling study. Lancet Infect Dis 2014; 14 : 1250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sonego M, Pellegrin MC, Becker G, Lazzerini M. Risk factors for mortality from acute lower respiratory infections (ALRI) in children under five years of age in low and middle-income countries: a systematic review and meta-analysis of observational studies. PLoS One 2015; 10 : e0116380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koyanagi A, Humphrey JH, Ntozini R et al. Morbidity among human immunodeficiency virus-exposed but uninfected, human immunodeficiency virus-infected, and human immunodeficiency virus-unexposed infants in Zimbabwe before availability of highly active antiretroviral therapy. Pediatr Infect Dis J 2011; 30 : 45–51. [DOI] [PubMed] [Google Scholar]
- 7. Gray DM, Zar HJ. Community-acquired pneumonia in HIV-infected children: a global perspective. Curr Opin Pulm Med 2010; 16 : 208–16. [DOI] [PubMed] [Google Scholar]
- 8. Chiappini E, Galli L, Tovo PA et al. Changing patterns of clinical events in perinatally HIV-1-infected children during the era of HAART. AIDS 2007; 21 : 1607–15. [DOI] [PubMed] [Google Scholar]
- 9. Gona P, Van Dyke RB, Williams PL et al. Incidence of opportunistic and other infections in HIV-infected children in the HAART era. JAMA 2006; 296 : 292–300. [DOI] [PubMed] [Google Scholar]
- 10. Mubiana-Mbewe M, Bolton-Moore C, Banda Y et al. Causes of morbidity among HIV-infected children on antiretroviral therapy in primary care facilities in Lusaka, Zambia. Trop Med Int Health 2009; 14 : 1190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kouakoussui A, Fassinou P, Anaky MF et al. Respiratory manifestations in HIV-infected children pre- and post-HAART in Abidjan, the Ivory Coast. Paediatr Respir Rev 2004; 5 : 311–5. [DOI] [PubMed] [Google Scholar]
- 12. Violari A, Cotton MF, Gibb DM et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008; 359 : 2233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Puthanakit T, Aurpibul L, Oberdorfer P et al. Hospitalization and mortality among HIV-infected children after receiving highly active antiretroviral therapy. Clin Infect Dis 2007; 44 : 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wamalwa D, Benki-Nugent S, Langat A et al. Survival benefit of early infant antiretroviral therapy is compromised when diagnosis is delayed. Pediatr Infect Dis J 2012; 31 : 729–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jaspan HB, Huang LC, Cotton MF et al. Bacterial disease and antimicrobial susceptibility patterns in HIV-infected, hospitalized children: a retrospective cohort study. PLoS One 2008; 3 : e3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ylitalo N, Brogly S, Hughes MD et al. Risk factors for opportunistic illnesses in children with human immunodeficiency virus in the era of highly active antiretroviral therapy. Arch Pediatr Adolesc Med 2006; 160 : 778–87. [DOI] [PubMed] [Google Scholar]
- 17. Smith KR, Samet JM, Romieu I, Bruce N. Indoor air pollution in developing countries and acute lower respiratory infections in children. Thorax 2000; 55 : 518–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lehman DA, Wamalwa DC, McCoy CO et al. Low-frequency nevirapine resistance at multiple sites may predict treatment failure in infants on nevirapine-based treatment. J Acquir Immune Defic Syndr 2012; 60 : 225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lehman DA, Chung MH, Mabuka JM et al. Lower risk of resistance after short-course HAART compared with zidovudine/single-dose nevirapine used for prevention of HIV-1 mother-to-child transmission. J Acquir Immune Defic Syndr 2009; 51 : 522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Emery S, Bodrug S, Richardson BA et al. Evaluation of performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay using primary subtype A, C, and D isolates from Kenya. J Clin Microbiol 2000; 38 : 2688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization. Pocketbook of hospital care for children: guidelines for the management of common childhood illnesses with limited resources. Geneva: : WHO Press; 2005. [Google Scholar]
- 22. Lanata CF, Rudan I, Boschi-Pinto C et al. Methodological and quality issues in epidemiological studies of acute lower respiratory infections in children in developing countries. Int J Epidemiol 2004; 33 : 1362–72. [DOI] [PubMed] [Google Scholar]
- 23. Sarkar S, Paul DK, Chakrabarti S et al. The Keith Edward scoring system: a case control study. Lung India 2009; 26 : 35–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization. The WHO child growth standards; 2006. Available at: http://www.who.int/childgrowth/en/. Accessed August 12, 2011. [Google Scholar]
- 25. Nelson LJ, Wells CD. Global epidemiology of childhood tuberculosis. Int J Tuberc Lung Dis 2004; 8 : 636–47. [PubMed] [Google Scholar]
- 26. Mbonye AK. Risk factors for diarrhoea and upper respiratory tract infections among children in a rural area of Uganda. J Health Popul Nutr 2004; 22 : 52–8. [PubMed] [Google Scholar]
- 27. Wamalwa DC, Obimbo EM, Farquhar C et al. Predictors of mortality in HIV-1 infected children on antiretroviral therapy in Kenya: a prospective cohort. BMC Pediatr 2010; 10 : 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kalish LA, McIntosh K, Read JS et al. Evaluation of human immunodeficiency virus (HIV) type 1 load, CD4 T cell level, and clinical class as time-fixed and time-varying markers of disease progression in HIV-1-infected children. J Infect Dis 1999; 180 : 1514–20. [DOI] [PubMed] [Google Scholar]
- 29. Shearer WT, Quinn TC, LaRussa P et al. Viral load and disease progression in infants infected with human immunodeficiency virus type 1. Women and Infants Transmission Study Group. N Engl J Med 1997; 336 : 1337–42. [DOI] [PubMed] [Google Scholar]
- 30. Rich KC, Fowler MG, Mofenson LM et al. Maternal and infant factors predicting disease progression in human immunodeficiency virus type 1-infected infants. Women and Infants Transmission Study Group. Pediatrics 2000; 105 : e8. [DOI] [PubMed] [Google Scholar]
- 31. Mofenson LM, Korelitz J, Meyer WA 3rd et al. The relationship between serum human immunodeficiency virus type 1 (HIV-1) RNA level, CD4 lymphocyte percent, and long-term mortality risk in HIV-1-infected children. National Institute of Child Health and Human Development Intravenous Immunoglobulin Clinical Trial Study Group. J Infect Dis 1997; 175 : 1029–38. [DOI] [PubMed] [Google Scholar]
- 32. Rouet F, Sakarovitch C, Msellati P et al. Pediatric viral human immunodeficiency virus type 1 RNA levels, timing of infection, and disease progression in African HIV-1-infected children. Pediatrics 2003; 112 : e289. [DOI] [PubMed] [Google Scholar]
- 33. Dickover RE, Dillon M, Leung KM et al. Early prognostic indicators in primary perinatal human immunodeficiency virus type 1 infection: importance of viral RNA and the timing of transmission on long-term outcome. J Infect Dis 1998; 178 : 375–87. [DOI] [PubMed] [Google Scholar]
- 34. Obimbo EM, Wamalwa D, Richardson B et al. Pediatric HIV-1 in Kenya: pattern and correlates of viral load and association with mortality. J Acquir Immune Defic Syndr 2009; 51 : 209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zaman K, Baqui AH, Yunus M et al. Association between nutritional status, cell-mediated immune status and acute lower respiratory infections in Bangladeshi children. Eur J Clin Nutr 1996; 50 : 309–14. [PubMed] [Google Scholar]
- 36. Rice AL, Sacco L, Hyder A, Black RE. Malnutrition as an underlying cause of childhood deaths associated with infectious diseases in developing countries. Bull World Health Organ 2000; 78 : 1207–21. [PMC free article] [PubMed] [Google Scholar]
- 37. Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA 2007; 298 : 1888–99. [DOI] [PubMed] [Google Scholar]
- 38. Peacock-Villada E, Richardson BA, John-Stewart GC. Post-HAART outcomes in pediatric populations: comparison of resource-limited and developed countries. Pediatrics 2011; 127 : e423–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Doshi S, Silk BJ, Dutt D et al. Household-level risk factors for influenza among young children in Dhaka, Bangladesh: a case-control study. Trop Med Int Health 2015; 20 : 719–29. [DOI] [PubMed] [Google Scholar]
- 40. Colosia AD, Masaquel A, Hall CB et al. Residential crowding and severe respiratory syncytial virus disease among infants and young children: a systematic literature review. BMC Infect Dis 2012; 12 : 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ram PK, Dutt D, Silk BJ et al. Household air quality risk factors associated with childhood pneumonia in urban Dhaka, Bangladesh. Am J Trop Med Hyg 2014; 90 : 968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kenya National Bureau of Statistics. Kenya Demographic and Health Survey 2008–09. KNBS and ICR Macro; 2010. Available at: http://www.dhsprogram.com/publications/publication-FR229-DHS-Final-Reports.cfm. Accessed September 15, 2015. [Google Scholar]