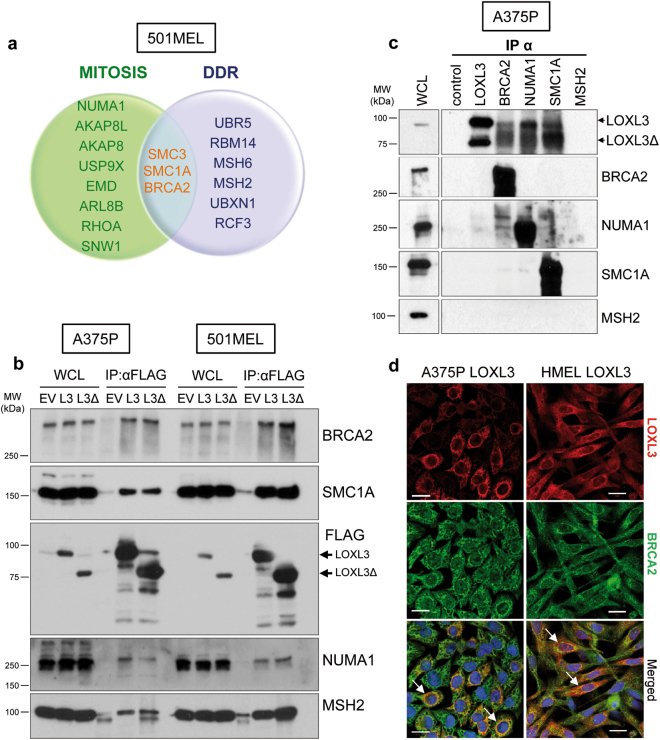
Fig. 4. LOXL3 physically interacts with proteins involved in genomic integrity maintenance.
(a) Venn diagram showing common proteins identified by mass spectrometry related to mitosis (green) and DDR (lilac) in 501MEL cells overexpressing LOXL3 or LOXL3Δ. Proteins involved in both processes are indicated in the middle (orange letters). (b) Interaction of selected proteins was confirmed by immunoblot analyses in A375P and 501MEL cells stably expressing empty vector (EV), FLAG-tagged LOXL3 (L3) or LOXL3Δ isoform (L3Δ) after FLAG immunoprecipitation. Whole-cell lysates (WCL) and eluted samples upon FLAG immunoprecipitation (IP: α-FLAG) were loaded and western blot analyses with the indicated antibodies were performed. Data are representative of three independent experiments. (c) Analysis of endogenous protein binding was performed in A375P whole-cell lysates (WCL) by individual immunoprecipitation (IP) with specific antibodies against BRCA2, NUMA1, SMC1A, and MSH2. WCL, control (IP without antibody) and eluates from the indicated samples were analyzed by western blot for the presence of LOXL3. MSH2 was not immunoprecipitated. MW of protein standards (kDa) is indicated (b, c). (d) Confocal images showing co-localization of 55 and 33% (Manders’ Co-localization Coefficient) of LOXL3 and BRCA2 in A375P and HMEL cells, respectively. Cell lines were stably transduced with FLAG-LOXL3, fixed and processed for BRCA2 (green) and FLAG (red) immunofluorescence. DAPI (blue) was used to stain DNA. White arrows in the merged images mark the subcellular location where BRCA2 and LOXL3 co-localize. Scale bar: 20 μm