Figure 2.
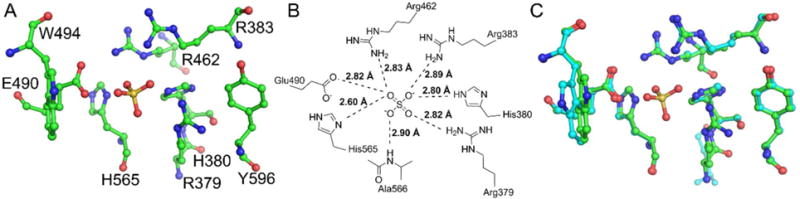
Active site of human Sts-1HP. A ball and stick diagram (A, carbon atoms colored green, nitrogen atoms colored blue, oxygen atoms colored red, and the sulfur atom is colored yellow) shows the active site residues of Sts-1HP around the phosphate binding pocket (marked by the sulfate in this structure). In addition to the conserved histidine residues and arginine residues, additional hydrogen bonding contacts are made to the sulfate by an additional arginine residue and a glutamate residue (B). The superposition of the human and murine Sts-1HP (C, overall RMSD = 0.35 Å) illustrates that the architecture of the active site is highly structurally conserved. The only observed difference is a slight change in orientation of the tryptophan (W494 in the human protein) at the edge of the active site.
