Figure 3.
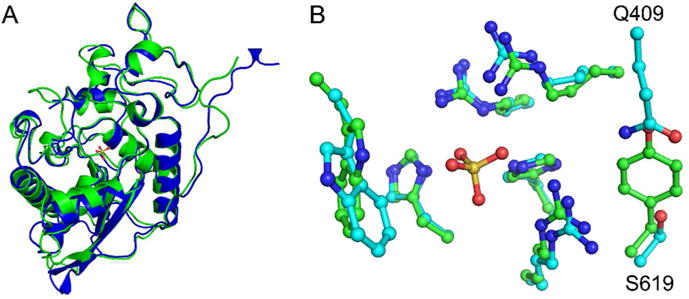
Structure of human Sts-2HP. The overall fold of human Sts-2HP is nearly identical to that of Sts-1HP. A superposition of the Sts-2HP protomer with the equivalent Sts-1HP protomer (A, Sts-1 is green and Sts-2 is blue, RMSD = 1.02 Å) shows only subtle differences in peripheral loop regions. Similarly, the active sites of the two proteins align well (B, Sts-1 has green carbon atoms, Sts-2 has cyan carbon atoms; in both structures the nitrogen atoms are blue, oxygen atoms are red and the sulfur atom is yellow) but do show some subtle, yet distinct differences. Whereas the conserved histidine and arginine residues line up well, a tyrosine and valine residue in Sts-1 (Y596 and V386) are replaced with a glutamine-serine (Q409 and S619) pair in Sts-2. It is these differences that likely account for the substrate selectivity of the Sts proteins.
