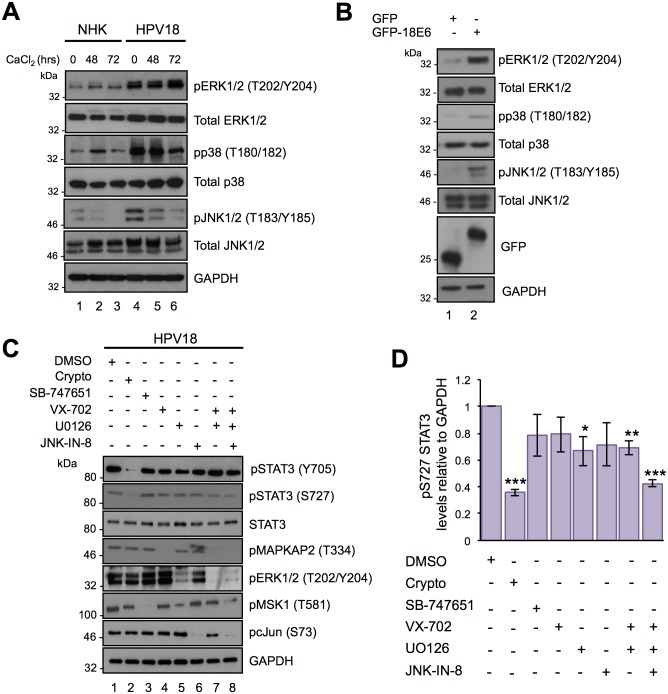
Fig 5. Identifying the protein kinases responsible for the S727 phosphorylation of STAT3 in HPV18 containing keratinocytes.
A) Representative western blot of keratinocytes subjected to high calcium differentiation analysed with antibodies specific for the total and phosphorylated forms of ERK1/2 (Thr202/Tyr204), p38 (Thr180/Tyr182) and JNK (Thr183/Tyr185). GAPDH served as a loading control. Data shown are representative of at least three independent biological repeats. B) Representative western blot of C33a cells transfected with GFP and GFP tagged HPV18 E6 and analysed using specific antibodies detecting phosphorylated and total forms of ERK1/2 (Thr202/Tyr204), p38 (Thr180/Tyr182) and JNK (Thr183/Tyr185). GAPDH served as a loading control. C) Representative western blots of HPV18 containing keratinocytes incubated with specific inhibitors of STAT3 (Crypto), MSK1 (SB-747651A; SB), p38 (VX-745; VX)), JNK (JNK-IN-8), MKK1/2 (UO126) or in combination as described in the methods and materials and examined with antibodies specific for phosphorylated and total STAT3. The phosphorylation status of substrate proteins pMSK1 (T581), pcJun (S73), pMAPKAPK2 (T334), and ERK1/2 (T202/Y204) demonstrated inhibitor efficacy and specificity. D) Quantification of the protein band intensities of (C) standardised to GAPDH and shown relative to the DMSO control. Bars represent the means ± standard deviation from at least three independent biological repeats. *P<0.05, **P<0.01, ***P<0.001 (Student’s t-test).