Abstract
Background and aims
Hepatocyte growth factor (HGF) has previously been associated with risk of stroke, coronary heart disease, and atherosclerosis. We hypothesized that higher circulating HGF is associated with greater progression of measures of atherosclerosis: coronary artery calcium (CAC) and carotid plaque.
Methods
Participants aged 45 to 84 years from the prospective cohort study Multi-Ethnic Study of Atherosclerosis had HGF measured at baseline (between 2000 and 2002) and were followed for progression of atherosclerosis for up to 12 years. CAC was measured at all five exams using the Agatston method. Mixed-effects models were used to examine the association of HGF and CAC progression among 6695 participants with available data. Relative risk regression was used to assess the association between HGF and new or additional carotid plaque between exams 1 and 5 in 3400 participants with available data. All point estimates were adjusted for potential confounding variables.
Results
Each standard deviation higher HGF at baseline was associated with 2.9 Agatston units/year greater CAC progression (95% CI: 1.6–4.2, p < 0.0001), and the magnitude of this association differed by race/ethnicity (p value for interaction by race = 0.003). Each standard deviation higher HGF at baseline was associated with a 4% higher risk of new or additional carotid plaque (95% CI: 1.01–1.08, p = 0.005).
Conclusions
Higher levels of HGF were significantly associated with greater progression of atherosclerosis in this large and diverse population. Circulating HGF continues to show promise as a potential clinical biomarker for cardiovascular disease.
Keywords: Hepatocyte growth factor, Atherosclerosis, Cardiovascular risk factors, Coronary artery calcium, Carotid plaque
1. Introduction
The protein hepatocyte growth factor (HGF) and its receptor c-MET are produced in response to tissue injury and are functional in tissue repair mechanisms. Their favorable effects in the heart and vasculature include anti-inflammatory, anti-fibrotic, and pro-angiogenic actions [1]. Because HGF is released in response to endothelial injury, circulating HGF has been proposed as a potential clinical biomarker for cardiovascular disease (CVD) [1].
In accordance with the hypothesis that HGF could serve as a biomarker for CVD, higher levels of circulating HGF are associated with stroke, coronary heart disease, and atherosclerosis [2,3]. However, the association of HGF with progression of atherosclerosis is unknown. Atherosclerosis burden is known to be dynamic, with progression and regression possible over time [4]. Aptly put by McEvoy et al., “[one measurement of atherosclerosis] can be thought of as a single point on an atherosclerosis vs. time curve, whereas progression correlates with the slope of that curve” [5].
Therefore, we sought to examine the relation between circulating HGF and progression of atherosclerosis using data from a large, multi-ethnic, population-based prospective cohort study: the Multi-Ethnic Study of Atherosclerosis (MESA). We hypothesized that circulating HGF is positively associated with progression of measures of atherosclerosis: coronary artery calcium (CAC) and carotid plaque.
2. Patients and methods
2.1. Study population
MESA is a prospective cohort study that was initiated to investigate the prevalence, correlates, and progression of subclinical CVD [6]. MESA recruited 6814 participants aged 45–84 and free of clinically recognized CVD from populations near six field centers in Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles, California; New York, New York; and Saint Paul, Minnesota. For exclusion purposes, CVD was defined as heart attack, angina, stroke, transient ischemic attack, heart failure, resuscitated cardiac arrest, or having undergone procedures related to CVD. Of the 6772 participants with serum HGF at baseline, we excluded individuals from all analyses if they 1) had an extreme value of HGF, defined as four or more standard deviations (SDs) beyond the mean (n = 31); 2) were subsequently found to have prevalent CVD at baseline (n = 4); or 3) had missing data for any variable included in analyses (n = 42). Our final sample size for statistical analyses was 6695. Carotid plaque analyses were additionally restricted to participants with both baseline and follow-up (exam 5) carotid ultrasound data (n = 3400 included in carotid plaque analyses). Exam 1 (baseline) occurred from 2000 to 2002, exam 2 from 2002 to 2004, exam 3 from 2004 to 2005, exam 4 from 2005 to 2007, and exam 5 from 2010 to 2012. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. The Institutional Review Boards at participating centers approved MESA and its ancillary studies, and all participants gave written informed consent.
2.2. Baseline measurements
2.2.1. Measurement of HGF
Venous blood was obtained from fasting participants. Serum separation was performed within 30 minutes of phlebotomy, and aliquots were subsequently stored at −70°C. Circulating levels of HGF protein were measured in serum using a quantitative sandwich enzyme-linked immunosorbent assay (ELISA) with the Quantikine Human HGF Immunoassay kit (R&D Systems, Minneapolis, Minnesota, USA). This method was validated by R&D systems, as specified in the package insert, and verified by the University of Minnesota laboratory that measured HGF for this study. The lower limit of detection was 40 pg/ml. The interassay laboratory coefficients of variation were 12.0, 8.0, and 7.4% at respective mean concentrations of 687, 2039, and 4080 pg/ml for lyophilized manufacturer’s controls, and 10.4% at a mean concentration of 688 pg/ml for an in-house pooled serum control.
2.2.2. Other baseline measurements
Sex, age, race/ethnicity, cigarette smoking status, and education were obtained via questionnaires, and medication use was assessed via a medication inventory. Body mass index (BMI) was calculated as weight over height squared (kg)/(m2). Resting blood pressure was measured three times in the seated position using a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon, Tampa, Florida, USA). The average of the last two measurements was used in analyses. Participants were asked to fast for at least eight hours before their exam. Serum glucose was assayed by a glucose oxidase method on the Vitros analyzer (Johnson & Johnson Clinical Diagnostics, Rochester, New York, USA). Diabetes was defined as use of insulin or other diabetes medication, self-reported physician diagnosis, or fasting glucose ≥126 mg/dL. Total cholesterol was measured in ethylenediaminetetraacetic (EDTA) plasma using a cholesterol oxidase method (Roche Diagnostics, Indianapolis, Indiana, USA) on a Roche COBAS FARA centrifugal analyzer. After precipitation of non-high-density lipoprotein cholesterol with magnesium/dextran, high-density lipoprotein cholesterol was also measured in EDTA plasma using the cholesterol oxidase method (Roche Diagnostics). Physical activity was determined by calculation of weekly metabolic-equivalent-of-task minutes of total walking, conditioning, and sports activity.
2.3. Measurement of outcomes: CAC progression and carotid plaque progression
2.3.1. CAC progression
The methodology used to measure CAC progression has been described in detail elsewhere [7]. Briefly, all participants (n = 6695) were scanned using noncontrast cardiac computed tomography during the baseline exam. By design, about half of the participants were scanned again during exam 2 (n = 2908), and the other half were scanned during exam 3 (n = 2764). The exam 4 selection strategy prioritized scanning participants without exam 3 scans (n = 1388 scanned during exam 4). The exam 5 selection strategy prioritized scanning participants with scans from exams 3 and/or 4 (n = 3264 scanned during exam 5). Because CAC scans are not meaningful after revascularization, we excluded scans that were obtained subsequent to coronary revascularization procedures that were performed after baseline (no participants had coronary revascularization at baseline due to exclusion criteria). Consistent calibration of scans was conducted using a “phantom” [8] and CAC scores were quantified using the Agatston method [9]. CAC progression was then measured in Agatston units/year.
2.3.2. New or additional carotid plaque
The methodology used to measure carotid plaque progression has been described in detail elsewhere [10]. Briefly, at baseline and exam 5, B-mode ultrasound images of the right and left common, bifurcation, and internal carotid artery segments were recorded on Super-VHS videotape with a Logiq 700 ultrasound system using the M12L transducer (General Electric Medical Systems). Carotid plaque was defined as a discrete, focal wall thickening ≥1.5 mm or focal thickening ≤50% greater than the surrounding IMT. Measurements of carotid plaque from baseline and exam 5 carotid ultrasound images were performed simultaneously. Carotid plaque score was defined as the number of carotid plaques (range 0–12) in the internal, bifurcation, and common segments of both carotid arteries. New or additional carotid plaque was defined as a binary variable (increase, no increase) based on any increase in carotid plaque score. Although uncommon (n = 30), if a participant had plaque regression between baseline and exam 5, they were categorized as “no increase,” as recommended elsewhere [5].
2.4. Statistical analyses
We performed analyses using SAS statistical software (version 9.4) and considered a p value <0.05 on a 2-tailed test as statistically significant. We presented baseline characteristics, mean CAC, and carotid plaque score by HGF tertile. Model 1 adjusted for basic demographics: age (continuous), race/ethnicity (non-Hispanic white American, Chinese American, African American, Hispanic American), and sex (male, female). Model 2 adjusted for all measured potential confounding variables: variables in Model 1 plus baseline values of BMI (continuous), smoking status (current, former, never), diabetes mellitus (yes, no), systolic blood pressure (continuous), antihypertensive medication use (yes, no), high-density lipoprotein cholesterol (continuous), total cholesterol (continuous), lipid-lowering medication use (yes, no), level of education (lower than high school level, high school level [ie, high school completed], some college education/technical school certificate or associate degree level, bachelor’s degree level, and graduate or professional school level), and physical activity (continuous). Model 2 in carotid plaque analyses additionally adjusted for time elapsed between exams 1 and 5 (continuous). By including cross-product terms in models, we tested for interactions of HGF with race/ethnicity.
2.4.1. CAC progression
CAC was modeled continuously on the raw scale in Agatston units. We used a mixed-effects model, as has previously been described in detail [7], to estimate the difference in average annual CAC progression (Agatston units/year) per one SD (259 pg/ml) increase in baseline HGF levels and corresponding 95% confidence intervals (CIs). Briefly, the analysis jointly models cross-sectional and longitudinal effects of covariates on CAC while accommodating participant-specific random slopes and intercepts. This approach offers three main benefits: 1) It allows us to control for baseline levels of CAC without inducing bias; 2) the assumption that data are missing completely at random is not required and, thus, risk of selection bias is mitigated; and 3) participants who did not attend all five exams can be included in the analysis, again lessening the risk of selection bias.
2.4.2 New or additional carotid plaque
We used relative risk regression [11] to calculate the risk of new or additional carotid plaque per one SD (259 pg/ml) higher baseline HGF and corresponding 95% CIs. Specifically, the probability of new or additional carotid plaque was modeled as a function of covariates using a generalized linear model with log link and binomial error distribution. In cases in which the model failed to converge with the binomial error, we substituted Gaussian error and used robust standard error estimates, as others have done [12]. We chose relative risk regression because the odds ratio is an overestimate of the relative risk when the outcome is not rare (i.e. >10%), as is the case here.
3. Results
The mean age of MESA participants at baseline was 62 years and ~50% were women. Baseline age, sex, race/ethnicity, level of physical activity, level of education, systolic blood pressure, use of antihypertensive or lipid-lowering medications, diabetes mellitus, smoking status, BMI, total cholesterol, and high-density lipoprotein cholesterol differed by HGF tertile, typically with a higher burden of CVD risk factors per increasing HGF tertile. Total cholesterol did not differ by HGF tertile. One participant had the maximum carotid plaque score of 12 at baseline and was thus excluded from carotid plaque analyses because there was no chance for progression. Those who attended exam 5, and were thus included in analyses of new or additional carotid plaque, on average had a lower burden of CVD risk factors and lower levels of HGF at baseline than all participants (Table 1). Mean CAC and carotid plaque score increased by exam and HGF tertile (Figs. 1 and 2).
Table 1.
Baseline characteristics of participants according to tertiles of hepatocyte growth factor, Multi-Ethnic Study of Atherosclerosis, 2000–2002.
| Baseline characteristics (means or prevalences unless otherwise stated) | All participants (n = 6695) | Attended exams 1 and 5 (n = 3400) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Hepatocyte growth factor tertile | Hepatocyte growth factor tertile | |||||
|
| ||||||
| <810 pg/ml (n = 2231) | 810–1011 pg/ml (n = 2232) | >1011 pg/ml (n = 2232) | <810 pg/ml (n = 1252) | 810–1011 pg/ml (n = 1162) | >1011 pg/ml (n = 986) | |
| Range of hepatocyte growth factor, pg/mL | 292–809 | 810–1011 | 1012–2152 | 343–808 | 809–1011 | 1012–2090 |
|
| ||||||
| Hepatocyte growth factor ± SD | 682 ± 93 | 906 ± 57 | 1227 ± 198 | 680 ± 93 | 905 ± 57 | 1201 ± 174 |
| Age, years ± SD | 59 ± 9 | 62 ± 10 | 65 ± 10 | 58 ± 9 | 61 ± 9 | 63 ± 10 |
| Male, % | 52 | 45 | 45 | 51 | 44 | 45 |
| Race/ethnicity | ||||||
| Non-Hispanic white American, % | 43 | 37 | 36 | 43 | 36 | 37 |
| Chinese American, % | 18 | 12 | 6 | 19 | 13 | 6 |
| African American, % | 27 | 29 | 26 | 26 | 29 | 24 |
| Hispanic American, % | 12 | 23 | 31 | 12 | 22 | 33 |
| Total moderate and vigorous physical activity, MET-minutes/week ± SD | 6231 ± 6086 | 5825 ± 6116 | 5222 ± 5425 | 6353 ± 5811 | 6068 ± 6409 | 5756 ± 5601 |
| More than high school education, % | 73 | 63 | 55 | 78 | 66 | 60 |
| Systolic blood pressure, mmHg ± SD | 122 ± 20 | 127 ± 21 | 131 ± 22 | 121 ± 19 | 125 ± 21 | 128 ± 21 |
| Antihypertensive medication use, % | 27 | 37 | 46 | 25 | 37 | 44 |
| Diabetes, % | 6 | 12 | 19 | 5 | 9 | 17 |
| Current smoker, % | 9 | 12 | 18 | 9 | 10 | 16 |
| Body mass index, kg/m2 ± SD | 27 ± 5 | 28 ± 5 | 30 ± 6 | 27 ± 5 | 28 ± 5 | 30 ± 5 |
| Lipid-lowering medication use, % | 14 | 16 | 19 | 13 | 17 | 20 |
| Total cholesterol, mg/dL ± SD | 194 ± 35 | 196 ± 36 | 192 ± 36 | 194 ± 34 | 197 ± 35 | 192 ± 36 |
| High-density lipoprotein cholesterol, mg/dL ± SD | 53 ± 16 | 51 ± 15 | 49 ± 14 | 53 ± 15 | 51 ± 15 | 49 ± 14 |
MET, metabolic equivalent task; SD, standard deviation.
Fig. 1.
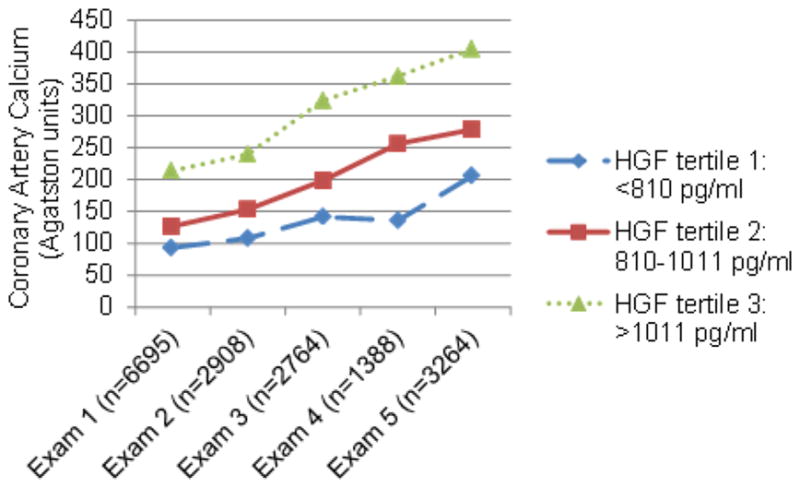
Mean coronary artery calcium by exam and HGF tertile.
Fig. 2.
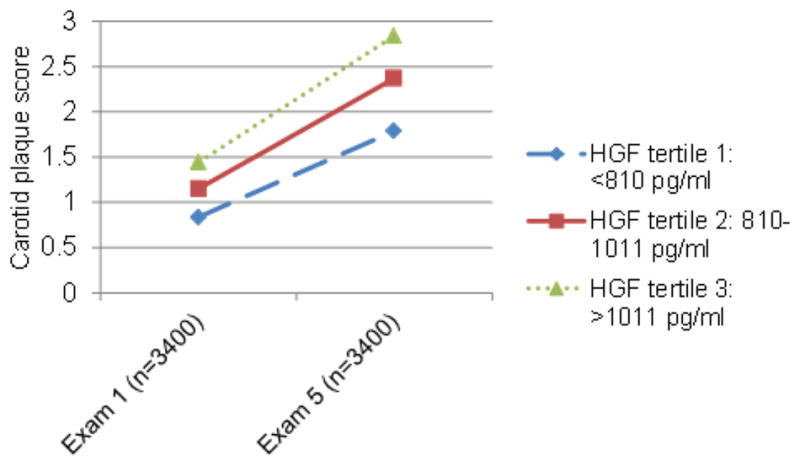
Mean carotid plaque score by exam and HGF tertile (analysis restricted to persons with carotid ultrasound data at both exams 1 and 5).
3.1. CAC progression
After adjustment for age, race/ethnicity, and sex, HGF was associated with CAC progression (difference in average annual progression per 1 SD higher baseline HGF 5.3; 95% CI: 4.0–6.6, p value < 0.0001) (Table 2). Adjustment for other potential confounding variables slightly attenuated the association between HGF and progression of atherosclerosis, but it remained statistically significant (difference in average annual progression 2.9; 95% CI: 1.6–4.2; p value <0.0001), and the magnitude of this association differed by race/ethnicity (p value for interaction by race 0.003). Each one SD higher HGF at baseline was significantly associated with 5.5 Agatston units/year greater CAC progression in African Americans (95% CI: 2.7–8.2; p value <0.0001) and 3.3 in non-Hispanic white Americans (95% CI: 1.2–5.4, p value 0.003), but not in in Chinese Americans (relative risk 1.9; 95% CI: −2.3–6.0; p value 0.4), or Hispanic Americans (relative risk 0.8; 95% CI: −1.4–3.1; p value 0.5).
Table 2.
Adjusted associations between one standard deviation increase in hepatocyte growth factor and coronary artery calcium progression (Agatston units/year), Multi-Ethnic Study of Atherosclerosis, 2000–2012.
| Statistical modela | Difference in average annual progression (95% confidence interval) | p value |
|---|---|---|
| All participants (n = 6695) | ||
| Model 1 | 5.3 (4.0, 6.6) | <0.0001 |
| Model 2 | 2.9 (1.6, 4.2) | <0.0001 |
| Non-Hispanic white Americans (n = 2587) | ||
| Model 1 | 5.7 (3.7, 7.8) | <0.0001 |
| Model 2 | 3.3 (1.2, 5.4) | 0.003 |
| Chinese Americans (n = 796) | ||
| Model 1 | 4.6 (0.4, 8.7) | 0.03 |
| Model 2 | 1.9 (−2.3, 6.0) | 0.4 |
| African Americans (n = 1837) | ||
| Model 1 | 7.7 (5.0, 10.4) | <0.0001 |
| Model 2 | 5.5 (2.7, 8.2) | <0.0001 |
| Hispanic Americans (n = 1475) | ||
| Model 1 | 2.5 (0.2, 4.7) | 0.03 |
| Model 2 | 0.8 (−1.4, 3.1) | 0.5 |
Mixed effects models with outcome coronary artery calcium progression (Agatston units/year) and predictor one standard deviation (259 pg/ml) increase of hepatocyte growth factor.
Model 1 - Adjusted for age at exam 1, race/ethnicity, and sex.
Model 2 - Adjusted for Model 1 plus baseline values of body mass index, smoking status, diabetes mellitus, systolic blood pressure, antihypertensive medication use, high-density lipoprotein cholesterol, total cholesterol, lipid medication use, level of education, and physical activity.
3.2. New or additional carotid plaque
After adjustment for age, race/ethnicity, and sex, each one SD higher HGF at baseline is associated with a 7% higher risk of new or additional carotid plaque (95% CI: 1.04–1.09; p value <0.0001) (Table 3). Adjustment for other potential confounding variables slightly attenuated the association between HGF and risk of new or additional plaque, but it remained statistically significant (relative risk 1.04; 95% CI: 1.01–1.08; p value 0.005), and this association did not differ by race/ethnicity (p value for interaction by race 0.3).
Table 3.
Adjusted relative risks for new or additional carotid plaque per one standard deviation increase in hepatocyte growth factor, Multi-Ethnic Study of Atherosclerosis, 2000–2012.
| Statistical modela | N with new or additional carotid plaque/total N in model | Relative risk (95% confidence interval) | p value |
|---|---|---|---|
| All participants | |||
| Model 1 | 1900/3400 | 1.07 (1.04, 1.09) | <0.0001 |
| Model 2 | 1.04 (1.01, 1.08) | 0.005 | |
| Non-Hispanic white Americans | |||
| Model 1 | 794/1335 | 1.05 (1.02, 1.09) | 0.003 |
| Model 2 | 1.04 (0.99, 1.09) | 0.1 | |
| Chinese Americans | |||
| Model 1 | 219/438 | 1.03 (0.93, 1.13) | 0.6 |
| Model 2 | 1.03 (0.92, 1.14) | 0.6 | |
| African Americans | |||
| Model 1 | 485/893 | 1.05 (0.98, 1.12) | 0.1 |
| Model 2 | 1.02 (0.95, 1.09) | 0.6 | |
| Hispanic Americans | |||
| Model 1 | 402/734 | 1.09 (1.05, 1.13) | <0.0001 |
| Model 2 | 1.09 (1.04, 1.15) | 0.001 | |
Relative risk regression with outcome new or additional carotid plaque and predictor one standard deviation (259 pg/ml) increase of hepatocyte growth factor.
Model 1 - Adjusted for age, race/ethnicity, and sex.
Model 2 - Adjusted for Model 1 plus baseline values of body mass index, smoking status, diabetes mellitus, systolic blood pressure, antihypertensive medication use, high-density lipoprotein cholesterol, total cholesterol, lipid medication use, time elapsed between visits 1 and 5, level of education, and physical activity.
4. Discussion
Our primary finding is that HGF was positively associated with progression of atherosclerosis – measured as CAC progression and new or additional plaque – in this large and diverse population. As far as we are aware, the current study is the first study to examine the association between HGF and progression of atherosclerosis, although mechanistic in vitro studies show the connection between HGF and CVD and support our epidemiological findings [13,14]. The association between HGF and CAC progression differed by race/ethnicity, whereas the association between HGF and risk of new or additional plaque did not.
The positive association between HGF and CAC progression has clinical implications. Take for instance, our finding that each SD increase in HGF at baseline was associated with 5.5 Agatston units greater CAC progression per year. Previous research found that a 5 unit annual change in CAC was associated with a 50% higher risk of coronary heart disease among those without CAC at baseline, and a 30% higher risk among those with CAC at baseline [15].
Reasons for racial/ethnic heterogeneity in the relationship between HGF and CAC progression remain unclear. Our team found similar racial/ethnic heterogeneity (i.e. stronger associations in non-Hispanic white Americans and African Americans than other races/ethnicities) in associations between HGF and coronary heart disease, and HGF and a single measurement of atherosclerosis [2]. Previous research has shown that the prevalence of CAC differs by race/ethnicity, with non-Hispanic white Americans typically having higher levels of CAC than other races/ethnicities [16]. A post-hoc analysis of the current study shows that non-Hispanic white Americans also had the highest rate of annual CAC progression (26.7 Agatston units per year) compared to Chinese Americans (19.0), African Americans (20.5), and Hispanic Americans (19.5), who all had similar rates of annual progression. While this study and others have found that CAC burden and progression differs by race/ethnicity, its predictive value for CVD does not [16–20].
It is not surprising that the association between HGF and CAC progression differs by race/ethnicity, whereas the association between HGF and risk of new or additional plaque does not. Research findings regarding coronary and carotid atherosclerosis do not always align, with carotid atherosclerosis (e.g. new or additional plaque) often a better predictor of stroke, and coronary atherosclerosis (e.g. CAC progression) often a better predictor of coronary heart disease [21–23]. It is also possible that the smaller sample size in our carotid plaque analyses precluded power to detect an interaction.
Higher baseline HGF was associated with a modest risk of new or additional carotid plaque, which was statistically significant but may not be clinically meaningful. Although it is known that one measure of carotid atherosclerosis predicts CVD events, there is not clear evidence that progression of carotid atherosclerosis predicts CVD events [24].
HGF has favorable effects in the heart and vasculature, which include anti-inflammatory, anti-fibrotic, and pro-angiogenic actions. HGF reduces inflammation by inducing IL-10 production and decreasing IL-8 and MCP-1 levels [1]. HGF inhibits fibrosis through neutralizing the powerful fibrotic properties of transforming growth factor β1 [25–30]. HGF promotes angiogenesis, which is of primary importance in repairing damage from cardiovascular disease, through action on endothelial and smooth muscle cells [1,31,32]. Thus, in the context of the positive effects of HGF on the heart and vasculature, it is a marker of atherosclerosis, not a cause.
In contrast, HGF is also implicated as a promoter of atherosclerosis and calcification [1,33]. HGF can stimulate pathological vascular calcification of smooth muscle cells via c-Met/Akt/Notch3 signalling [13]. Also, HGF may help form new blood vessels during plaque development, which contributes to the progression of atherosclerosis [14].
In summary, HGF is likely positively associated with progression of atherosclerosis both because it might promote atherosclerosis, but also because it is released to repair and protect tissue in response to atherosclerosis. It is unclear how large a role the positive versus negative effect of HGF on the heart and vasculature plays in driving the association between HGF and progression of atherosclerosis.
A limitation of this study is the potential for selection bias. The carotid plaque analyses were limited to participants who returned for exam 5, which may be a biased sample. And, although participants with no follow-up contributed to baseline characterization in our CAC analyses, they do not contribute to the progression rate. On average, people who participated in MESA past the baseline exam had a lower burden of CVD risk factors and lower levels of HGF at baseline than all MESA participants. This selective attrition could have created a downward bias, meaning that it is possible that the true association between HGF and progression of atherosclerosis is stronger than we observed [34]. Finally, it is worth noting that we are measuring circulating rather than tissue-specific HGF. However, these two values are correlated [35].
Strengths of this study include the prospective design; the large, population-based multi-ethnic sample with a wide geographic distribution in the United States; and the highly standardized assessment of a broad array of CVD risk factors.
In conclusion, HGF was positively associated with progression of atherosclerosis – measured as CAC progression and new or additional carotid plaque – in this large and diverse population. Circulating HGF – which has previously been shown to be associated with risk of stroke, coronary heart disease, and atherosclerosis – continues to show promise as a potential clinical biomarker for CVD.
Highlights.
Hepatocyte growth factor was associated with progression of atherosclerosis.
This association differed by race/ethnicity in some instances.
Hepatocyte growth factor shows promise as a biomarker for cardiovascular disease.
Acknowledgments
Financial support
Dr. Bell is supported by the NIH T32 Training Grant HL07111-40. This study was supported by the National Institutes of Health (NIH; grant numbers N01 HC95159, N01 HC95160, N01 HC95161, N01 HC95162, N01 HC95163, N01 HC95164, N01 HC95165, N01 HC95166, N01 HC95167, and N01 HC95168); National Heart, Lung, and Blood Institute (NHLBI) at NIH (grant number N01 HC95169); and National Center for Research Resources at NIH (grant numbers UL1 TR000040 and UL1 TR001079). Funding for adhesion protein levels was provided by the NHLBI at NIH (grant number R01 HL98077).
Role of the funding source: The funding source had no involvement in the study design, collection, analysis or interpretation of data, in the writing of the report or in the decision to submit the manuscript for publication.
We thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. MESA is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with MESA investigators.
Footnotes
Conflict of interest
Dr. Matthew Budoff receives a grant from General Electric. Dr. Stein has a patent related to carotid thickness and arterial age, assigned to Wisconsin Alumni Research Foundation (WARF) and receives royalties. The other authors have nothing to disclose.
Author contributions
Study design: EJB, SJB. Data collection: MYT, NQH, MB, JFP JHS, SJB. Data analysis: EJB, PAD, NBL, MB, JFP, JHS, SJB. Outcome adjudication: MB, JFP, JHS. Manuscript draft: All authors contributed, read and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gallo S, Sala V, Gatti S, Crepaldi T. Cellular and molecular mechanisms of HGF/Met in the cardiovascular system. Clin Sci. 2015;129:1173–1193. doi: 10.1042/CS20150502. [DOI] [PubMed] [Google Scholar]
- 2.Bielinski SJ, Berardi C, Decker PA, Larson NB, Bell EJ, et al. Hepatocyte growth factor demonstrates racial heterogeneity as a biomarker for coronary heart disease. Heart. 2017;103:1185–1193. doi: 10.1136/heartjnl-2016-310450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell EJ, Larson NB, Decker PA, Pankow JS, Tsai MY, et al. Hepatocyte growth factor is positively associated with risk of stroke: the MESA (Multi-Ethnic Study of Atherosclerosis) Stroke. 2016;47:2689–2694. doi: 10.1161/STROKEAHA.116.014172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295:1556–1565. doi: 10.1001/jama.295.13.jpc60002. [DOI] [PubMed] [Google Scholar]
- 5.McEvoy JW, Blaha MJ, Defilippis AP, Budoff MJ, Nasir K, et al. Coronary artery calcium progression: an important clinical measurement? A review of published reports. J Am Coll Cardiol. 2010;56:1613–1622. doi: 10.1016/j.jacc.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 6.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 7.Gassett AJ, Sheppard L, McClelland RL, Olives C, Kronmal R, et al. Risk factors for long-term coronary artery calcium progression in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2015;4:e001726. doi: 10.1161/JAHA.114.001726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 9.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 10.Tattersall MC, Gassett A, Korcarz CE, Gepner AD, Kaufman JD, et al. Predictors of carotid thickness and plaque progression during a decade: the Multi-Ethnic Study of Atherosclerosis. Stroke. 2014;45:3257–3262. doi: 10.1161/STROKEAHA.114.005669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lumley T, Kronmal R, Ma S. Relative Risk Regression in Medical Research: Models, Contrasts, Estimators, and Algorithms. [accessed 16 February 2018];UW Biostatistics Working Paper Series. 2006 Jul; Working Paper 293 http://biostats.bepress.com/uwbiostat/paper293/
- 12.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;115:2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Wang T, Yan J, Jiagbogu N, Heideman DA, et al. HGF/c-Met signalling promotes Notch3 activation and human vascular smooth muscle cell osteogenic differentiation in vitro. Atherosclerosis. 2011;219:440–447. doi: 10.1016/j.atherosclerosis.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Wilkinson FL, Kirton JP, Jeziorska M, Iizasa H, et al. Hepatocyte growth factor and c-Met expression in pericytes: implications for atherosclerotic plaque development. J Pathol. 2007;212:12–19. doi: 10.1002/path.2155. [DOI] [PubMed] [Google Scholar]
- 15.Budoff MJ, Young R, Lopez VA, Kronmal RA, Nasir K, et al. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2013;61:1231–1239. doi: 10.1016/j.jacc.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bild DE, Detrano R, Peterson D, Guerci A, Liu K, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 17.Lee TC, O’Malley PG, Feuerstein I, Taylor AJ. The prevalence and severity of coronary artery calcification on coronary artery computed tomography in black and white subjects. J Am Coll Cardiol. 2003;41:39–44. doi: 10.1016/s0735-1097(02)02618-9. [DOI] [PubMed] [Google Scholar]
- 18.Budoff MJ, Yang TP, Shavelle RM, Lamont DH, Brundage BH. Ethnic differences in coronary atherosclerosis. J Am Coll Cardiol. 2002;39:408–412. doi: 10.1016/s0735-1097(01)01748-x. [DOI] [PubMed] [Google Scholar]
- 19.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;113:30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 20.Diez Roux AV, Detrano R, Jackson S, Jacobs DR, Jr, Schreiner PJ, et al. Acculturation and socioeconomic position as predictors of coronary calcification in a multiethnic sample. Circulation. 2005;112:1557–1565. doi: 10.1161/CIRCULATIONAHA.104.530147. [DOI] [PubMed] [Google Scholar]
- 21.Folsom AR, Kronmal RA, Detrano RC, O’Leary DH, Bild DE, et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA) Arch Intern Med. 2008;168:1333–1339. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newman AB, Naydeck BL, Ives DG, Boudreau RM, Sutton-Tyrrell K, et al. Coronary artery calcium, carotid artery wall thickness, and cardiovascular disease outcomes in adults 70 to 99 years old. Am J Cardiol. 2008;101:186–192. doi: 10.1016/j.amjcard.2007.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lester SJ, Eleid MF, Khandheria BK, Hurst RT. Carotid intima-media thickness and coronary artery calcium score as indications of subclinical atherosclerosis. Mayo Clin Proc. 2009;84:229–233. doi: 10.4065/84.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Volzke H, et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet. 2012;379:2053–2062. doi: 10.1016/S0140-6736(12)60441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura T, Matsumoto K, Mizuno S, Sawa Y, Matsuda H, et al. Hepatocyte growth factor prevents tissue fibrosis, remodeling, and dysfunction in cardiomyopathic hamster hearts. Am J Physiol Heart Circ Physiol. 2005;288:H2131–2139. doi: 10.1152/ajpheart.01239.2003. [DOI] [PubMed] [Google Scholar]
- 26.Taniyama Y, Morishita R, Nakagami H, Moriguchi A, Sakonjo H, et al. Potential contribution of a novel antifibrotic factor, hepatocyte growth factor, to prevention of myocardial fibrosis by angiotensin II blockade in cardiomyopathic hamsters. Circulation. 2000;102:246–252. doi: 10.1161/01.cir.102.2.246. [DOI] [PubMed] [Google Scholar]
- 27.Ueki T, Kaneda Y, Tsutsui H, Nakanishi K, Sawa Y, et al. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med. 1999;5:226–230. doi: 10.1038/5593. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi E, Sasamura H, Mifune M, Shimizu-Hirota R, Kuroda M, et al. Hepatocyte growth factor regulates proteoglycan synthesis in interstitial fibroblasts. Kidney Int. 2003;64:1179–1188. doi: 10.1046/j.1523-1755.2003.00202.x. [DOI] [PubMed] [Google Scholar]
- 29.Mizuno S, Matsumoto K, Li MY, Nakamura T. HGF reduces advancing lung fibrosis in mice: a potential role for MMP-dependent myofibroblast apoptosis. FASEB J. 2005;19:580–582. doi: 10.1096/fj.04-1535fje. [DOI] [PubMed] [Google Scholar]
- 30.Azuma J, Taniyama Y, Takeya Y, Iekushi K, Aoki M, et al. Angiogenic and antifibrotic actions of hepatocyte growth factor improve cardiac dysfunction in porcine ischemic cardiomyopathy. Gene Ther. 2006;13:1206–1213. doi: 10.1038/sj.gt.3302740. [DOI] [PubMed] [Google Scholar]
- 31.Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, et al. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grant DS, Kleinman HK, Goldberg ID, Bhargava MM, Nickoloff BJ, et al. Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci U S A. 1993;90:1937–1941. doi: 10.1073/pnas.90.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ritman EL, Lerman A. The dynamic vasa vasorum. Cardiovasc Res. 2007;75:649–658. doi: 10.1016/j.cardiores.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weuve J, Tchetgen Tchetgen EJ, Glymour MM, Beck TL, Aggarwal NT, et al. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology. 2012;23:119–128. doi: 10.1097/EDE.0b013e318230e861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu Y, Hojo Y, Ikeda U, Shimada K. Production of hepatocyte growth factor during acute myocardial infarction. Heart. 2000;83:450–455. doi: 10.1136/heart.83.4.450. [DOI] [PMC free article] [PubMed] [Google Scholar]


