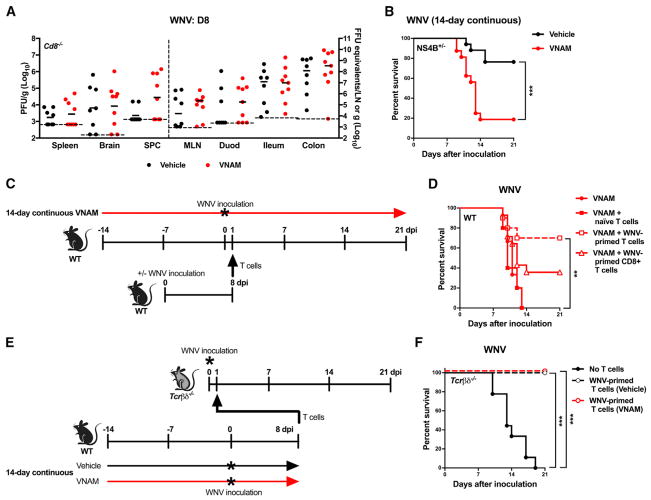
Figure 4. Oral Abx Treatment Impacts Optimal T Cell Responses during WNV Infection.
(A–F) Cd8−/− (A), NS4B+/− Tg (B), WT (D), and Tcrβδ−/− (F) mice were untreated or treated with vehicle or VNAM for 14 days continuously and inoculated with WNV as in Figure 1B or as illustrated in (C) and (E). Tissues (A) were harvested at day 8 after virus inoculation, and WNV burden was assessed as in Figure 2 (all comparisons were not statistically significant (p > 0.05, Mann-Whitney test). Survival curves (B, D, and F) were compared using the log-rank test with a Bonferroni correction (**p < 0.01 and ***p < 0.001). Results were combined from two to four independent experiments: vehicle (n = 8), VNAM (n = 9) (A); vehicle (n = 17), VNAM (n = 16) (B); VNAM (n = 15), VNAM + naive T cells (n = 10), VNAM + WNV-primed T cells (n = 10), VNAM + WNV-primed CD8+ T cells (n = 14) (D); and no T cells (n = 9), WNV-primed T cells (vehicle) (n = 8), WNV-primed T cells (VNAM) (n = 8) (F).