Scheme 3.
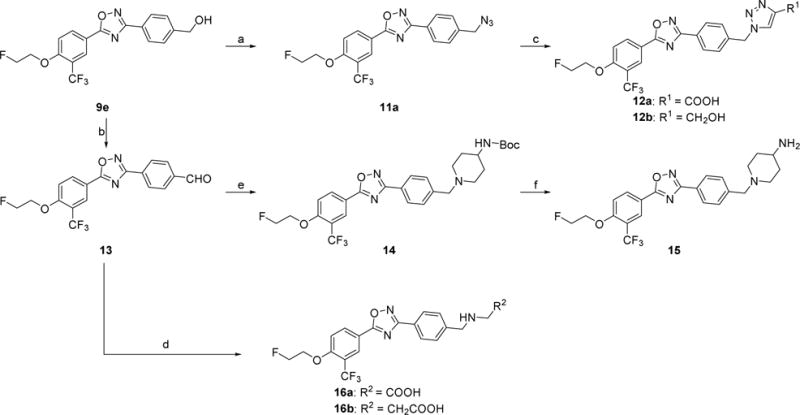
Syntheses of 12a–b, 15, and 16a–b. Reagents and conditions: (a) DPPA, DBU, toluene, 0 °C-RT; (b) oxalyl chloride, DMSO, CH2Cl2, triethylamine, −78 °C-RT; (c) propiolic acid or propargyl alcohol, CuSO4•5H2O, sodium ascorbate, THF, H2O, RT; (d) NaBH3CN, amines, acetic acid, methanol, RT; (e) 4-(N-Boc-amino)piperidine, NaBH(OAc)3, acetic acid, 1,2-dichloroethane, RT; (f) 4 M HCl in dioxane, RT.
