Abstract
Rationale:
Venous thromboembolism may result from prolong immobilization following intracerebral hemorrhage. Massive pulmonary embolism with associated right heart failure is life-threatening, requiring treatment with anticoagulants or even thrombolytic agents. However, these drugs are contraindicated after a recent hemorrhagic episode, as they may induce further hemorrhage. There are no guidelines for treatment in these circumstances.
Patient concerns:
A 57-year-old man experienced massive pulmonary embolism and shock 18 days after an intracerebral hemorrhage.
Diagnoses:
Tachycardia and high D-dimer (21.27 mg/L fibrinogen-equivalent units) were noted. Chest computed tomography showed bilateral pulmonary trunk embolism.
Interventions:
Heparinization were used and activated partial thromboplastin time therapeutic range was 50 to 70 seconds. Fortunately, shock status and shortness of breath improved two days later. Continuing high dose Rivaroxaban was administrated for three weeks.
Outcomes:
There was no recurrent intracranial hemorrhage (ICH) following treatment for three-weeks with high-dose and one-year with standard dose of rivaroxaban. This report presents a treatment option in the management of these difficult clinical situations.
Lessons:
The combination of unfractionated heparin infusion and continuing non-Vitamin K antagonist oral anticoagulants use could manage life-threatening pulmonary embolism following recent ICH. Theoretically, the use of NOAC is a safer strategy if the patient with previous history of major ICH.
Keywords: critical care, intracranial hemorrhage, non-Vitamin K antagonist oral anticoagulants, pulmonary embolism
1. Introduction
A treatment dilemma exists in patients who experience life-threatening venous thromboembolism (VTE) following recent severe brain injury. Clinicians must decide which anticoagulants to use in patients who have had a recent cerebral hemorrhage. We report a case of severe pulmonary embolism with right heart failure and shock following recent intracranial hemorrhage without surgical intervention. A strategy of unfractionated heparin infusion and non-Vitamin K antagonist oral anticoagulants (NOAC) was used with good outcome, and did not result in secondary hemorrhagic events, or recurrent thromboembolism. All studies using NOAC for VTE and stroke prevention in patients with atrial fibrillation, showed a lower incidence of intracranial hemorrhage (ICH), than with heparin and warfarin.[1] Therefore, it is reasonable and safe to use NOAC to treat VTE in patients who have had a hemorrhagic episode recently.
2. Case presentation
A 57-year-old man experienced sudden onset of shortness of breath for several hours, requiring emergency intubation for impending respiratory failure with low oxygen saturation. Tachycardia and high D-dimer (21.27 mg/L fibrinogen-equivalent units) were noted. Electrocardiography showed new inverted T-waves in V1 to V4 leads. Chest computed tomography (CT) showed bilateral pulmonary trunk embolism (Fig. 1A–D). The patient had a medical history of hypertension, and 18 days prior to this episode, he had presented with a left hemiparesis and was diagnosed with a right thalamic hemorrhage (ICH) with rupture into the ventricle (Fig. 2A, B). He was transferred to our hospital for advanced treatment of massive pulmonary embolism (PE).
Figure 1.
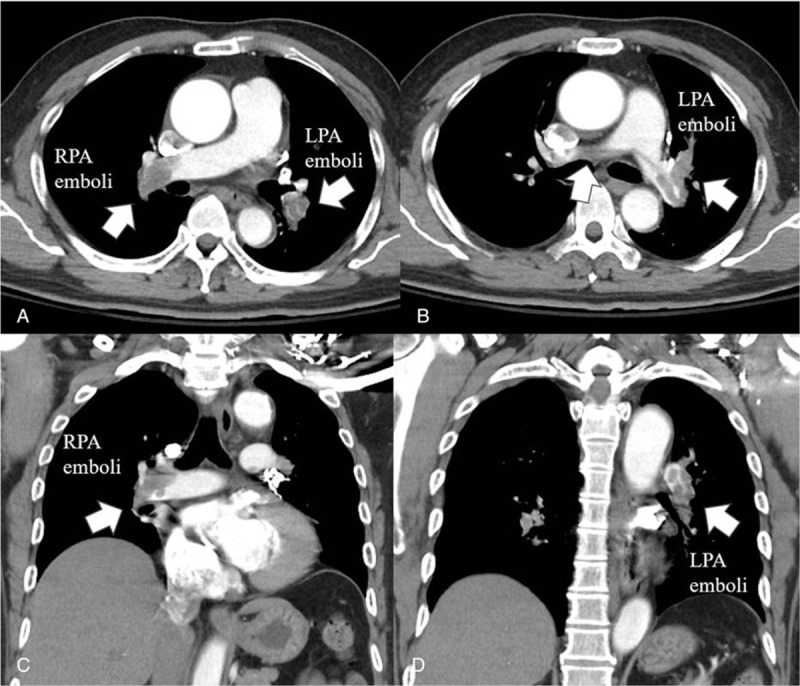
Chest computed tomography. A: Cross-section. Right pulmonary arterial emboli (left lower white arrow) and left pulmonary arterial emboli (right lower white arrow) were noted. B: Cross-section: pulmonary trunk emboli (central white arrow) and left pulmonary arterial trunk and branch emboli (right lower white arrow) were noted. C: Longitudinal section: white arrow showing right pulmonary arterial emboli. D: Longitudinal section: white arrow showing left pulmonary arterial emboli.
Figure 2.
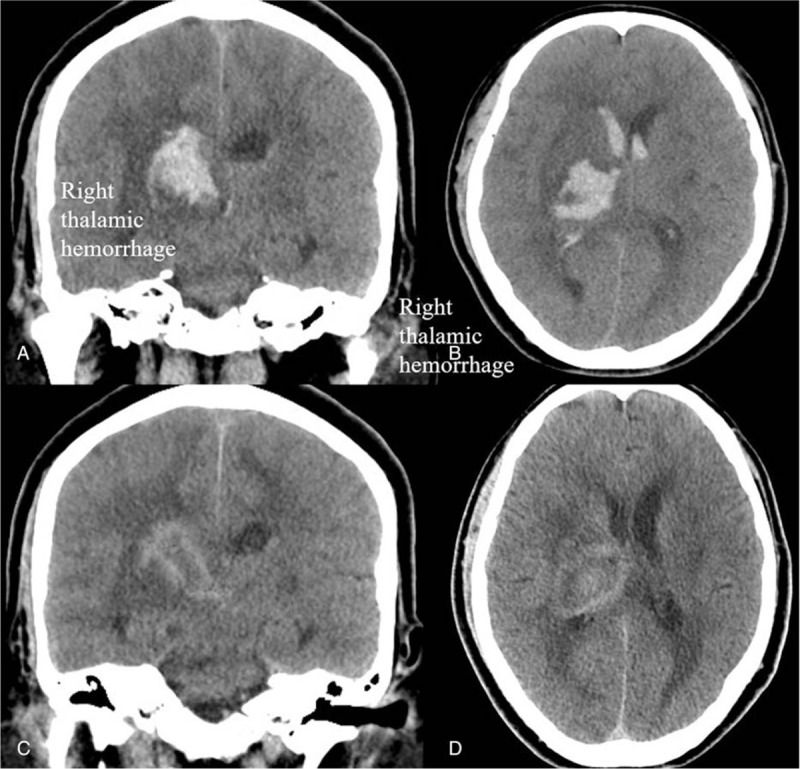
Brain computed tomography (CT). A, B: Cross-section and longitudinal section: right thalamic hemorrhage with rupture into ventricle with some midline shifting. C, D: Cross-section and longitudinal section: Following CT did not present recurrent hemorrhage after high dose rivaroxaban.
On arrival at our emergency department, he experienced a state of shock with a systolic blood pressure of 70 mmHg. Transthoracic echocardiography showed a dilated right ventricle (RV) with poor RV performance (tricuspid annular plane systolic excursion <10 mm), and RV to left ventricle (LV) ratio was greater than 1.3. Acute kidney injury and impaired liver function were noted and considered to be related to the hypotensive episode. Adequate hydration was achieved, and high-dose intravenous dopamine was used to manage for the acute kidney injury and PE related right ventricular failure. For life threatening PE, systemic thrombolysis was not suitable for this patient due to the recent ICH. Unfractionated heparin (UFH) was considered to use because we could control therapeutic range and could convert the effect of anticoagulation if massive bleeding happened. UFH infusion was commenced to maintain activated partial thromboplastin time 50 to 70 seconds. The hemodynamics improved after treatment, and no new neurological signs were detected. The patient's renal function also returned to normal. We converted to a direct oral anticoagulant 3 days later but did not consider warfarin because of high risk of ICH. Our insurance only covered rivaroxaban for PE, and we therefore commenced a high-dose rivaroxaban regimen (15 mg twice per day) for 3 weeks. Duplex vein test and venography showed possible deep vein thrombosis (DVT) of the left leg. An inferior vena cava (IVC) filter was not implanted because the patient was already using anticoagulants, and there was patent venous flow. One week later, lung perfusion scan showed a perfusion defect. Two weeks later, follow-up brain CT did not show any signs of recurrent ICH (Fig. 2C, D). After 3-week high dose regimen and discharge, standard dose rivaroxaban (20 mg per day) was used for 1 year. During the 1-year follow-up period, the patient did not have any recurrence of DVT, PE, or ICH.
3. Discussion
ICH is the most severe form of stroke, and survivors suffer high rates of functional disability, resulting in an increased likelihood of thromboembolic complications such as DVT, PE, and myocardial infarction.[2] A previous report of patients with ICH demonstrated a prevalence of 2% for PE and 1% for DVT.[1] Massive PE is a life-threatening condition requiring treatment with anticoagulants or even thrombolytic agents in high-risk cases. However, anticoagulants and thrombolytic agents are contraindicated after a recent hemorrhagic episode. The treatment of a life-threating PE in the setting of a recent ICH presents a difficult problem. In addition, while pharmacological or mechanical prophylaxis for VTE post neurosurgery or brain injury may be considered, the optimal method remains controversial.[3] One study showed that the majority of DVTs occurred within the first week after neurosurgical procedures and the use of early subcutaneous heparin (at either 24 or 48 hours) was associated with a 43% reduction in the development of a lower-extremity DVT, with no increase in surgical site hemorrhage.[4] The use of IVC filters may be necessary in the short-term prevention of PE if anticoagulants are contraindicated.[4]
In recent years, non-vitamin K oral anticoagulants (NOAC) became a safe alternative treatment for low risk patients with PE and DVT. All studies using NOAC for VTE and stroke prevention in patients with atrial fibrillation (AF), showed a lower incidence of major bleeding, including ICH, than with heparin and warfarin.[1] It is thus reasonable and safe to use NOAC to treat VTE in patients who have had a hemorrhagic episode. However, in randomized trials, those patients who had experienced recent hemorrhagic events were excluded. It therefore remains controversial as to when to restart anticoagulants, and which treatment to use. According to a large registry, patients who had VTE <14 days from the hemorrhagic event had an increased risk for rebleeding or death.[5] In the large registry recording patients with AF, the optimal timing of restarting treatment was 7 to 8 weeks after ICH.[6] Oral anticoagulants were better than antiplatelet agents, due to the lower incidence of recurrent ischemic events, as well as higher survival benefit.[7] Another meta-analysis describing the restarting of anticoagulants following ICH, was associated with a lower risk of thromboembolic complications, and a similar risk of ICH recurrence.[8] However, no large randomized studies have been performed for anticoagulants use in the patient with life-threating VTE following ICH.
It is well known that medical therapy is generally feasible for a non-massive PE. However, the treatment of a massive and potentially life-threatening PE can require thrombolytic agents, which can have dangerous side effects in patients with extensive brain damage, especially if secondary to hemorrhage.[9] One case report reported prolonged UFH infusion followed by warfarin, and described a successful uneventful outcome.[10] In our case, we used NOAC because of the lower probability of recurrent ICH. There was no recurrence of either hemorrhagic or embolic events following treatment for 3-weeks with high-dose and 1-year with standard dose of rivaroxaban. This report presents a treatment option in the management of these difficult clinical situations.
4. Conclusion
The combination of UFH infusion and NOAC use may be used to manage life-threatening PE following recent ICH. Theoretically, the use of NOAC is a safer strategy if the patient with previous history of major ICH. Further investigations and controlled trials for uncommon cases of ICH and acute PE are still needed.
Author contributions
Conceptualization: Wei-Chieh Lee.
Data curation: Wei-Chieh Lee.
Formal analysis: Wei-Chieh Lee.
Investigation: Wei-Chieh Lee.
Methodology: Wei-Chieh Lee.
Project administration: Wei-Chieh Lee.
Software: Wei-Chieh Lee.
Supervision: Wei-Chieh Lee.
Validation: Wei-Chieh Lee.
Visualization: Wei-Chieh Lee.
Writing – original draft: Wei-Chieh Lee.
Writing – review and editing: Hsiu-Yu Fang.
Footnotes
Abbreviations: CT = computed tomography, DVT = deep vein thrombosis, ICH = intracranial hemorrhage, IVC = inferior vena cava, LV = left ventricle, NOAC = non-Vitamin K antagonist oral anticoagulant, PE = pulmonary embolism, RV = right ventricle, UFH = unfractionated heparin, VTE = venous thromboembolism.
HYF has also equal contribution as correspondence author.
Sources of funding: None.
Disclosures: None.
Human rights statements and informed consent: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later revisions. The patient and her family agree to publish this case report.
All authors declare that they have no conflict of interest.
References
- [1].Lee LH. DOACs – advances and limitations in real world. Thromb J 2016;14(suppl):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Goldstein JN, Fazen LE, Wendell L, et al. Risk of thromboembolism following acute intracerebral hemorrhage. Neurocrit Care 2009;10:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lee K, Rincon F. Pulmonary complications in patients with severe brain injury. Crit Care Res Pract 2012;2012:207–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Guyatt GH, Akl EA, Crowther M, et al. American College of Chest Physicians Antithrombotic Therapy, Prevention of Thrombosis Panel. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012;141(suppl):7S–47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nieto JA, Bruscas MJ, Ruiz-Ribo D, et al. RIETE Investigators. Acute venous thromboembolism in patients with recent major bleeding. The influence of the site of bleeding and the time elapsed on outcome. J Thromb Haemost 2006;4:2367–72. [DOI] [PubMed] [Google Scholar]
- [6].Pennlert J, Overholser R, Asplund K, et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke 2017;48:314–20. [DOI] [PubMed] [Google Scholar]
- [7].Nielsen PB, Larsen TB, Skjøth F, et al. Restarting anticoagulant treatment after intracranial hemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality, and bleeding: a nationwide cohort study. Circulation 2015;132:517–25. [DOI] [PubMed] [Google Scholar]
- [8].Murthy SB, Gupta A, Merkler AE, et al. Restarting anticoagulant therapy after intracranial hemorrhage: a systematic review and meta-analysis. Stroke 2017;48:1594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Merli GJ. Pulmonary embolism in medical patients: improved diagnosis and the role of low-molecular-weight heparin in prevention and treatment. J Thromb Thrombolysis 2004;18:117–25. [DOI] [PubMed] [Google Scholar]
- [10].Oneglia C, Gualeni A. Pulmonary embolism after brain hemorrhage in a hypertensive patient: the therapeutic dilemma. J Thromb Thrombolysis 2008;25:231–4. [DOI] [PubMed] [Google Scholar]


