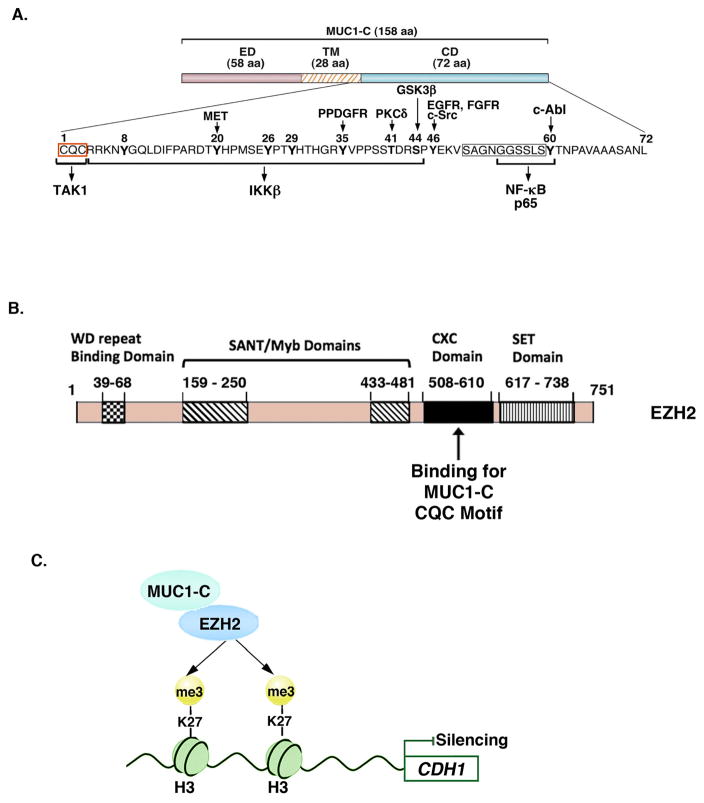
Figure 2. MUC1-C binds directly to EZH2 and promotes EZH2-induced H3K27 trimethylation.
A. Structure of the MUC1-C subunit, which includes a 58-aa extracellular domain (ED) and a 28-aa transmembrane domain (TM). The MUC1-C 72-aa cytoplasmic domain (CD) includes a CQC motif located immediately following the TM region that is necessary for MUC1-C homodimerization in the response to oxidative stress and for nuclear import 39, 54, 108. The CQC motif is the target for the GO-203 inhibitor. The remainder of the MUC1-C cytoplasmic domain is an intrinsically disordered region (IDR). IDRs have been identified in other oncoproteins, such as p53, that function as nodes for the integration of signaling cascades and are also prevalent in transcription factors and the transcriptional coactivators, p300 and CBP 109, 121. The MUC1-C cytoplasmic domain IDR is modified by diverse kinases and, as highlighted, interacts directly with multiple effectors of the inflammatory NF-κB p65 pathway 39, 108. The MUC1-C cytoplasmic domain CQC and the SAGNGGSSLS (boxed) motifs also bind directly to TCF4 and β-catenin, respectively, with activation of the WNT pathway. B. Schema of the EZH2 protein and the indicated domains. The MUC1-C CQC motif binds directly to the EZH2 CXC domain 35. C. MUC1-C forms a complex with EZH2, enhances EZH2 occupancy on the CDH1 promoter, and thereby increases H3K27 trimethylation with repression of E-cadherin expression 35.