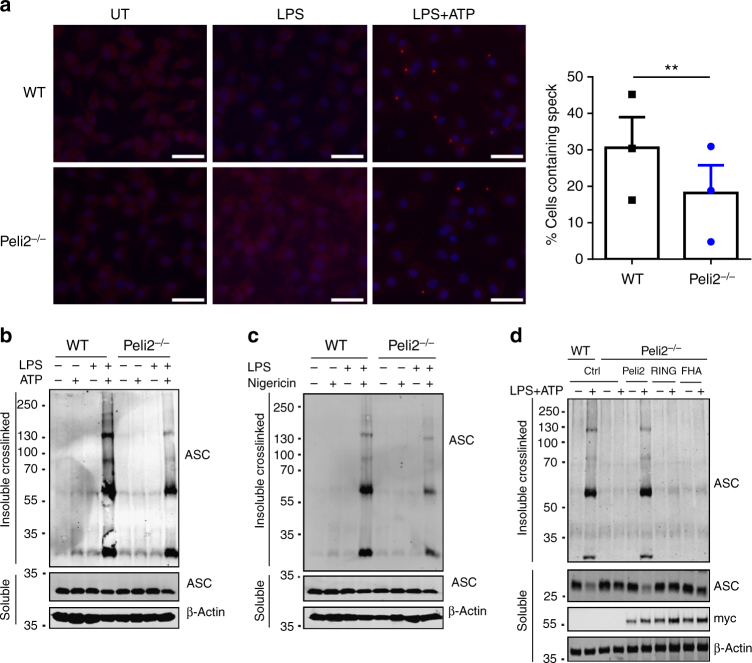
Fig. 6.
Pellino2 mediates NLRP3-dependent oligomerization of ASC. a Immunofluorescence staining of ASC in WT and Peli2−/− BMDMs that were left untreated (UT) or treated with 100 ng/ml LPS for 3 h and further stimulated with ATP for 30 min. ASC specks were detected by immunostaining using anti-ASC antibody and anti-rabbit Alexa Fluor 568 (red) and cells were counter stained with nuclei-staining DAPI. The histogram quantitates the percentage of cells that exhibit ASC speck formation. (scale bar = 100 μm). b, c Immunoblot analysis of ASC in chemically cross-linked NP-40 insoluble fractions and in NP-40 soluble fractions from cell lysates of WT and Peli2−/− BMDMs stimulated with 100 ng/ml LPS for 3 h with or without further treatment with b 2.5 mM ATP, or c 5 mM Nigericin for 30 min. β-Actin was used as loading controls. d WT and Peli2−/− BMDMs were infected with MSCV as control (Ctrl) or with MSCV containing an expression construct encoding myc-tagged murine Pellino2 (Peli2), Pellino2 RING mutant (RING), or Pellino2 FHA mutant (FHA). Immunoblot analysis of ASC in chemically cross-linked NP-40 insoluble fractions and in NP-40 soluble fractions from cell lysates of MSCV-infected cells treated with 100 ng/ml LPS for 3 h followed by 2.5 mM ATP for 30 min. The expression of the Pellino2 constructs was measured by immunoblotting with an anti-myc antibody. **p < 0.01 (paired, two-tailed Student’s t-test). Data are the mean ± s.e.m. of three independent experiments (a, right panel) or biological replicates that are representative of three independent experiments (a left panel, b, c, d)