Fig. 7.
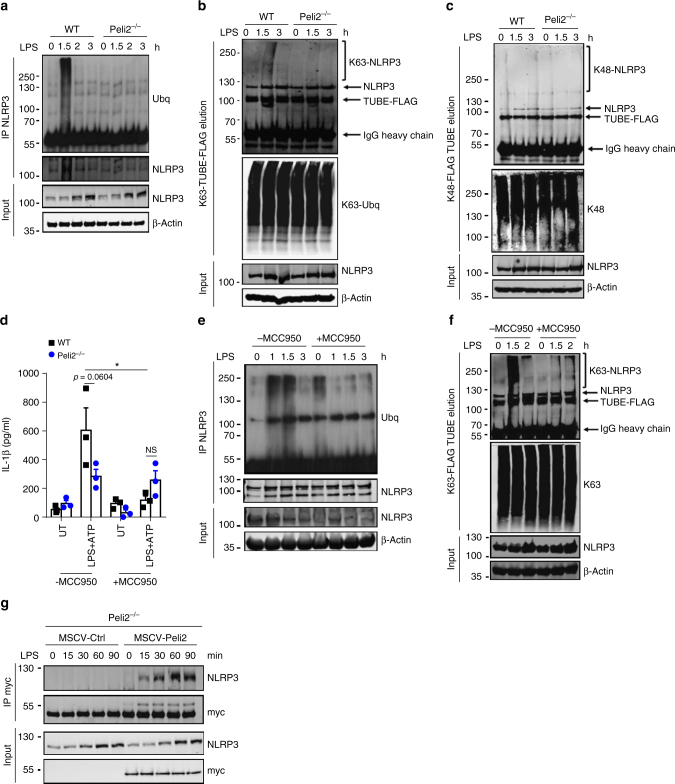
Pellino2 mediates LPS-induced ubiquitination and activation of NLRP3. a-c Immunoblot analysis of NLRP3 and ubiquitin in cell lysates (Input) and immunoprecipitated (IP) NLRP3 samples (a) or NLRP3 and K63-linked ubiquitin (K63-ubq) in K63-TUBE-FLAG elution and cell lysates (b) or NLRP3 and K48-ubq in K48-TUBE-FLAG elution and cell lysates (c) from WT and Peli2−/− BMDMs treated with a 100 ng/ml LPS for the indicated times. d ELISA of IL-1β in medium from WT and Peli2−/− BMDMs treated with 100 ng/ml LPS for 2 h followed by sequential treatment with 1 μM MCC950 for 1 h and 2.5 mM ATP for 30 min. UT, untreated. e, f Immunoblot analysis of ubiquitin and NLRP3 in lysates (Input) and immunoprecipitated (IP) NLRP3 samples (e) or NLRP3 and K63-ubq in K63-TUBE-FLAG elution and cell lysates (f) from WT and Peli2−/− BMDMs pre-treated with 1 μM MCC950 for 1 h followed by treatment with 100 ng/ml LPS for the indicated times. g Peli2−/− BMDMs were infected with MSCV as control (MSCV-Ctrl) or with MSCV containing an expression construct encoding myc-tagged murine Pellino2 (MSCV-Peli2). Immunoblot analysis of NLRP3 and myc in lysates (Input) and immunoprecipitated (IP) myc samples from MSCV-infected cells treated with 100 ng/ml LPS for the indicated times. β-Actin was used as loading controls. *p < 0.05 (paired, two-tailed Student’s t-test). NS, not significant. Data are biological replicates that are representative of three independent experiments (a-c, e-g) or mean ± s.e.m. of three independent experiments (d)