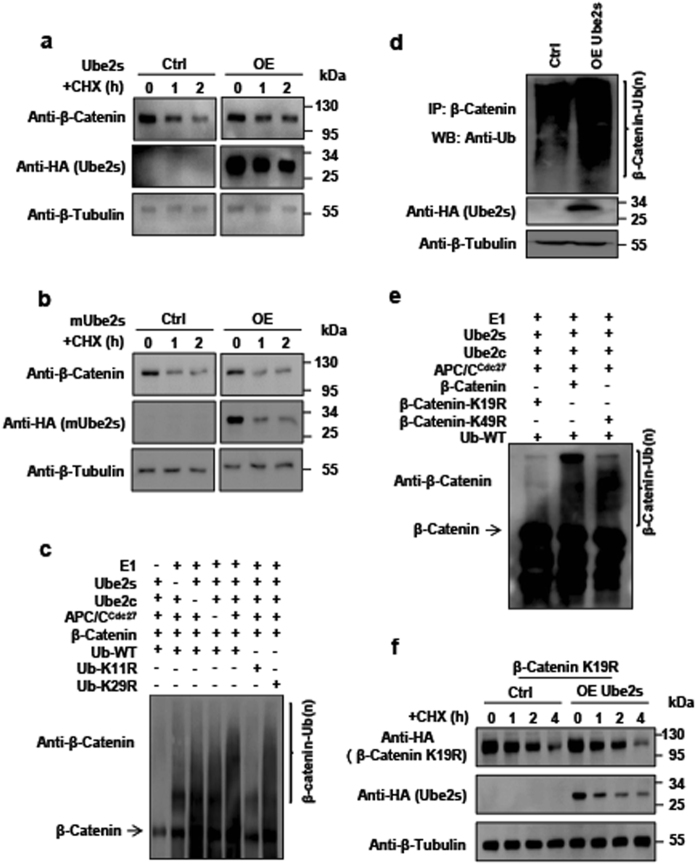
Fig. 2. Ube2s ubiquitinated β-Catenin to enhance its stability.
a Ube2s overexpression inhibited β-Catenin degradation. The construct overexpressing Ube2s was transfected into mES cells. The cells were treated with 12.5 µg/ml CHX for the indicated hours. b Ube2s-C95S mutant failed in stabilizing β-Catenin. mES cells were transfected with the construct expressing Ube2s-C95S. Experimental design and procedures are as described in a. Ctrl: vector transfection control; OE: overexpression. c Ube2s-mediated β-Catenin ubiquitination in vitro. The reaction components for in vitro ubiquitination assay include purified His6-tagged Ub, the E3 APCCdc27 complex co-IP by Cdc27 from mES cells, and purified E1 (Uba1), Ube2c, Ube2s, and β-Catenin proteins. The reaction solution was analyzed by western blot with an anti-β-Catenin antibody. d Ube2s promoted β-Catenin ubiquitination in vivo in mES cells. mES cells were transfected with a construct expressing Ube2s; 3 µM MG132 was added to treat cells for 15 h. Whole cell proteins were prepared for co-IP analysis with an anti-β-Catenin antibody, followed by western blotting with an anti-Ub antibody. e In vitro ubiquitination assay to identify β-Catenin-K19 as the ubiquitination site by Ube2s. Wild-type β-Catenin, β-Catenin-K19R, and β-Catenin-K49R were purified, respectively, for the in vitro ubiquitination assay. f Ube2s failed in stabilizing β-Catenin-K19R. The construct overexpressing Ube2s and empty vector were respectively co-transfected with the plasmid expressing β-Catenin-K19R into mES cells. Similar experimental procedure was employed with a. The data presented are based on three independent repeats