Table 2. The effect of solvent a .
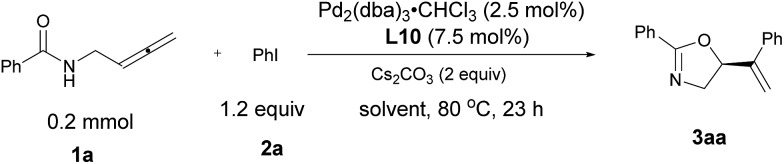
| ||||
| Entry | Solvent |
3aa
|
Recovery of 1a b (%) | |
| Yield b (%) | ee c (%) | |||
| 1 d | CH3CHCl2 | 60 | 64 | 30 |
| 2 e | CH3CCl3 | 24 | 67 | 64 |
| 3 d | THF | 6 | n.d. f | 94 |
| 4 | DME | 80 | 10 | 3 |
| 5 | MTBE | 59 | 52 | — |
| 6 | Dioxane | 54 | 46 | 34 |
| 7 | PhCH3 | 68 | 79 | 5 |
| 8 | o-Xylene | 69 | 87 | 25 |
| 9 | m-Xylene | 66 | 88 | 27 |
| 10 | p-Xylene | 71 | 87 | 24 |
| 11 g | Benzene | 69 | 85 | 27 |
| 12 h | Benzene | 83 i | 94 | — |
aUnless indicated otherwise, the experiments were performed with 1a (0.2 mmol), 2a (0.24 mmol), Pd2(dba)3·CHCl3 (0.005 mmol), L10 (0.015 mmol), and Cs2CO3 (0.4 mmol) in solvent (2 mL) for 23 h at 80 °C under Ar atmosphere.
bThe yield and recovery were determined by 1H NMR analysis using 1,3,5-trimethylbenzene as the internal standard.
cThe ee values were determined by HPLC analysis.
dThe reaction was conducted at 70 °C.
eReaction time: 22.5 h.
fn.d. = not determined.
gReaction time: 21 h.
hThe reaction was conducted with 1a (0.5 mmol), 2a (1.0 mmol), Pd2(dba)3·CHCl3 (0.0125 mmol), L10 (0.0375 mmol), and Cs2CO3 (1.0 mmol) in benzene (6 mL) for 0.5 h at rt, then 39 h at 90 °C under Ar atmosphere.
iIsolated yield.
