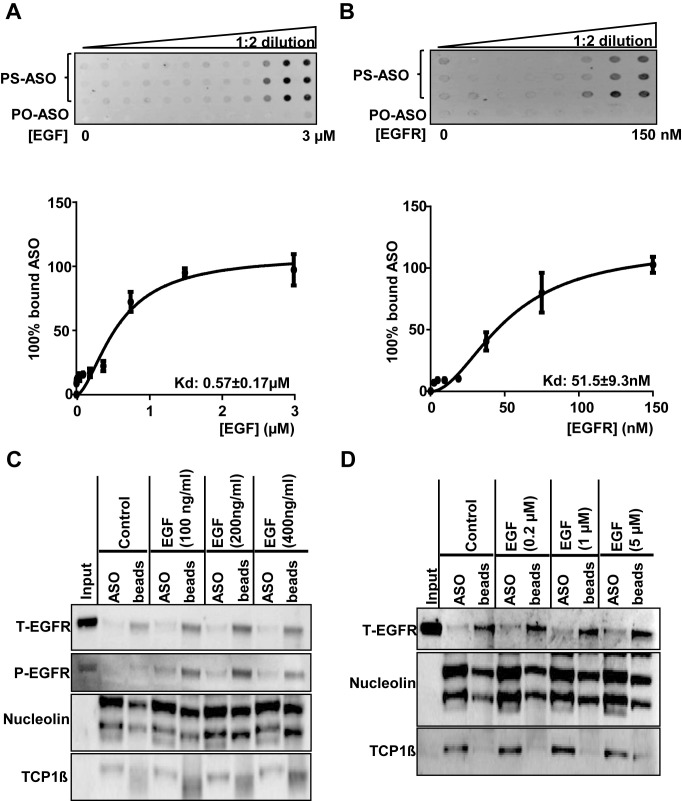
Figure 3.
PS-ASOs interact with EGFR more tightly than with EGF. (A and B) The membrane binding assay for EGF or EGFR and PS-ASOs. EGF at concentrations ranging from 3 nM to 3 μM or purified recombinant EGFR protein at concentrations ranging from 5 nM to 150 nM were incubated with PS-ASOs for 1 h at 37°C and the samples were loaded on a Hybond ECL nitrocellulose membrane. The signal intensities retained in nitrocellulose membrane for the protein bound form of the FITC-labeled 2′-MOE gapmer PS-ASO (PS-ASO, IONIS ID 256903) or phosphodiester ASO (PO-ASO) were quantified and the binding curves for EGF (A) and EGFR (B) were plotted using Prism. The error bars represent standard deviations from three experiments; (C) EGFR (total EGFR, T-EGFR) was blotted after PS-ASO affinity selection as shown in Figure 1, except that cell lysates were from A431 cells treated with indicated concentrations of EGF. The same blot was probed sequentially with antibodies to phosphorylated EGFR (P-EGFR), Nucleolin, and TCP1β. The experiments were repeated at least three times and representative results are shown; (D) EGFR (total EGFR, T-EGFR) was blotted after affinity selection as shown in Figure 1, except that the bound proteins were eluted by PS-ASOs in the presence of EGF at indicated concentrations. The same blot was probed sequentially with different antibodies against other ASO-binding proteins (Nucleolin and TCP1β). The experiments were repeated at least three times and representative results are shown.