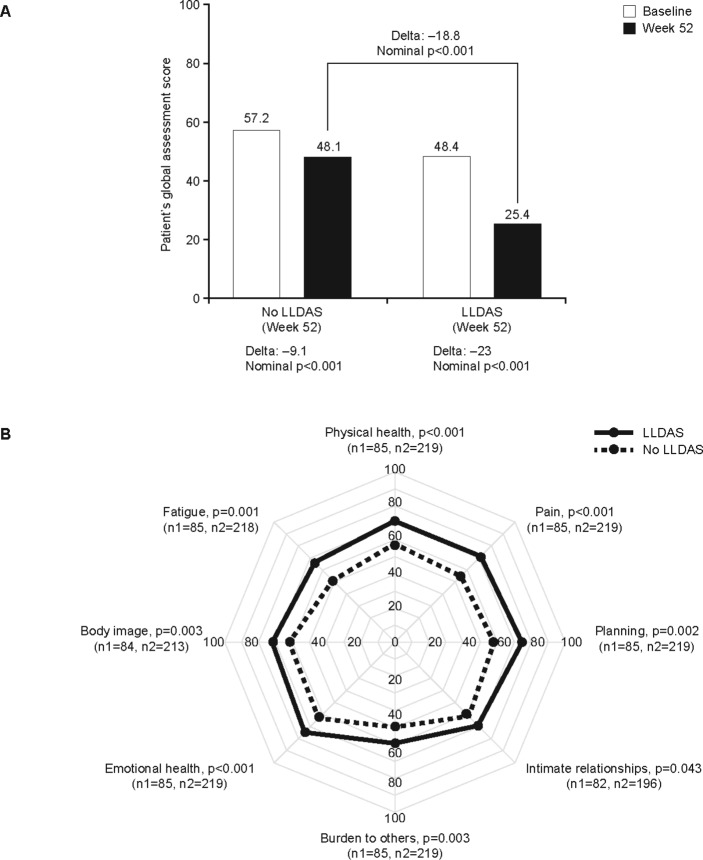
Figure 2.
Association of LLDAS with PROs for pooled patients with active SLE treated with placebo or anifrolumab. (A) Mean PaGA scores at baseline and Week 52 by LLDAS attainment at Week 52. (B) Mean LupusQOL domain scores at Week 52 by LLDAS attainment at Week 52. The nominal p-values and delta for comparing the difference in mean scores between patients in LLDAS and those who did not attain LLDAS at Week 52 were based on an ANCOVA test adjusted for treatment, randomisation stratification factors and respective baseline domain scores. Nominal p-values for comparing baseline with Week 52 PaGA scores were based on a Wilcoxon signed rank test. ANCOVA, analysis of covariance; LLDAS, Lupus Low Disease Activity State; PaGA, Patient ’s Global Assessment; PROs, patient-reported outcome; QOL, Quality of Life.