Figure 1. Loss of indigenous intestinal microbiota leads to vulnerability to C. difficile infection.
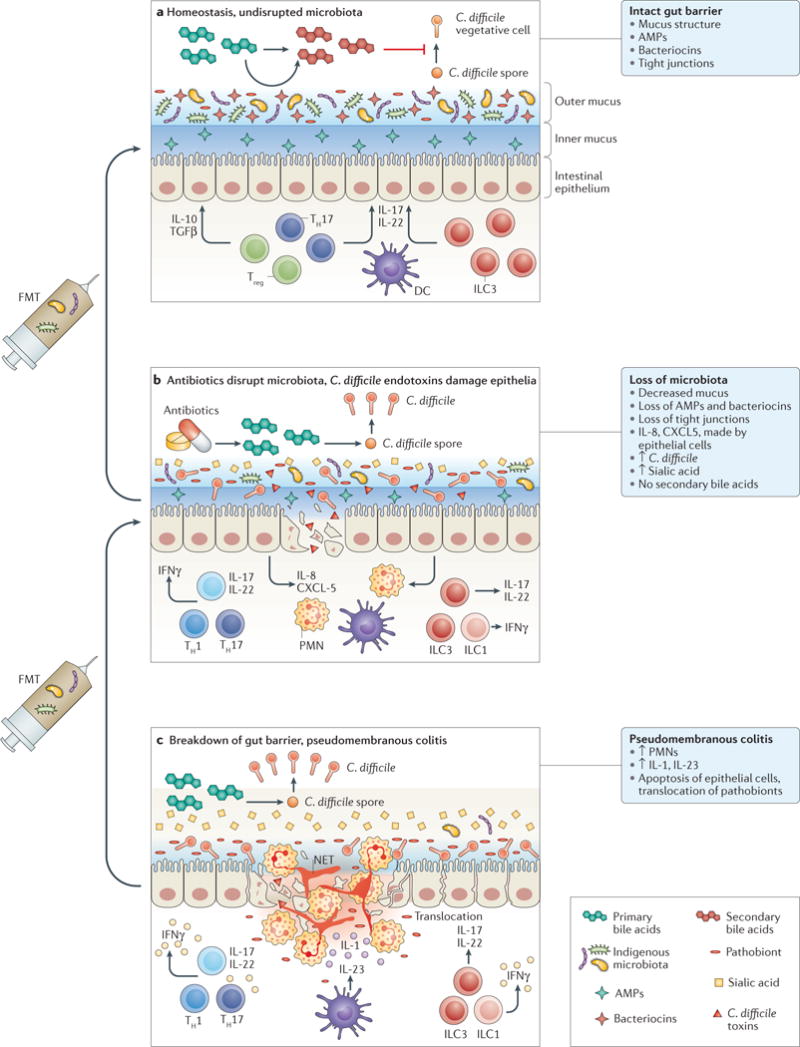
a At homeostasis, the indigenous gut microbiota is compartmentalized within the intestinal lumen. The gut barrier is maintained by organized mucus, antimicrobial peptides (AMPs) produced by the host and the microbiota, and tight junctions between the epithelial cells. Gut microbiota provides tonic stimulation to the mucosal immune system, leading to production of different cytokines, such as transforming growth factor (TGF)β and IL-22, which help to fortify the gut barrier defences. Competition for nutrients is intense. Gut microbiota also carry out transformation of primary bile acids, which lead to a secondary bile acid composition that is inhibitory to C. difficile spore germination and growth. b Antibiotic treatment suppresses most indigenous gut microbiota, although many drug-resistant microorganisms, which are also more likely to assume pathogenic roles themselves, remain intact. More nutrients, such as free sialic acid, become available to C. difficile. Primary bile acids are no longer converted to secondary bile acids, and the total bile acid composition becomes more favourable to C. difficile spore germination and growth. Production of mucus and AMPs is decreased. C. difficile enterotoxins lead to weakening of tight junctions and epithelial cell death. Neutrophils are recruited by chemokines, such as IL-8 and CXCL5. c Severe pseudomembranous colitis results from breakdown of gut barrier and translocation of residual microbiota. This step leads to activation of the inflammasome and production of IL-1. Massive recruitment of neutrophils occur, which form the pseudomembranes. Neutrophils release their DNA to form neutrophil extracellular traps (NETs), which form temporary patches in the broken gut barrier. T cells and innate lymphocytes produce more pro-inflammatory cytokines in response to loss of microbiota compartmentalization, which might further damage the gut epithelium and gut barriers. Faecal microbiota transplantation (FMT) restores the normal composition of gut microbiota. Severe pseudomembranous colitis typically requires multiple sequential applications of FMT. DC, dendritic cell; ILC, innate lymphoid cell; PMN, polymorphonuclear leukocyte; TH, T helper cell; Treg, regulatory T cell.
