Abstract
Background
To date, evidence for the efficacy of fecal microbiota transplantation (FMT) in recurrent Clostridium difficile infection (CDI) has been limited to case series and open-label clinical trials.
Objective
To determine the efficacy and safety of FMT for treatment of recurrent CDI.
Design
Randomized, controlled, double-blind clinical trial. (ClinicalTrials.gov: NCT01703494)
Setting
Two academic medical centers.
Patients
46 patients who had 3 or more recurrences of CDI and received a full course of vancomycin for their most recent acute episode.
Intervention
Fecal microbiota transplantation with donor stool (heterologous) or patient’s own stool (autologous) administered by colonoscopy.
Measurements
The primary end point was resolution of diarrhea without the need for further anti-CDI therapy during the 8-week follow-up. Safety data were compared between treatment groups via review of adverse events (AEs), serious AEs (SAEs), and new medical conditions for 6 months after FMT. Fecal microbiota analyses were performed on patients’ stool before and after FMT and also on donors’ stool.
Results
In the intention-to-treat analysis, 20 of 22 patients (90.9%) in the donor FMT group achieved clinical cure compared with 15 of 24 (62.5%) in the autologous FMT group (P = 0.042). Resolution after autologous FMT differed by site (9 of 10 vs. 6 of 14 [P = 0.033]). All 9 patients who developed recurrent CDI after autologous FMT were free of further CDI after subsequent donor FMT. There were no SAEs related to FMT. Donor FMT restored gut bacterial community diversity and composition to resemble that of healthy donors.
Limitation
The study included only patients who had 3 or more recurrences and excluded those who were immunocompromised and aged 75 years or older.
Conclusion
Donor stool administered via colonoscopy seemed safe and was more efficacious than autologous FMT in preventing further CDI episodes.
Primary Funding Source
National Institute of Diabetes and Digestive and Kidney Diseases.
Clostridium difficile infection (CDI) is the most common health care–associated infection in U.S. hospitals, with approximately 453 000 infections and 29 000 deaths in 2011 (1). Antibiotics are frequently ineffective (2, 3), with recurrence rates of 15% to 35% after a first episode and up to 65% after treatment of a second recurrence (4, 5). Recurrences are clinically challenging and are typically treated with prolonged courses of antibiotics, which maintain and exacerbate intestinal dysbiosis (6). Fecal microbiota transplantation (FMT) restores the normal composition of gut microbiota and is recommended when antibiotics fail to clear the infection (3). However, the evidence for FMT rests largely on case series and several open-label clinical trials that have suggested cure rates of 81% to 100% in recurrent CDI (7–12). To date, there has not been an adequately controlled and blinded trial of FMT for CDI treatment. Furthermore, the optimal method for administering FMT has not been determined. Evidence suggests that colonoscopic delivery has advantages in terms of efficacy (9), safety (13), and patient acceptance and tolerability (14) compared with administration via the nasoenteric route. We therefore performed a double-blind, randomized, controlled study of colonoscopic FMT for treatment of recurrent CDI. Cure rates and adverse events (AEs) were compared between donor FMT and autologous FMT (given as a “placebo”) in patients who had at least 3 CDI recurrences.
Methods
Design Overview
This prospective, dual-center, double-blind, randomized, controlled trial compared FMT using donor stool or the patient’s own stool administered by colonoscopy. Patients treated with autologous FMT whose CDI relapsed during the 8-week follow-up were offered FMT using donor stool. Those who underwent donor FMT and had relapse were offered repeated FMT using stool from a different donor.
Patients were enrolled between 15 November 2012 and 10 March 2015 at 2 academic hospitals: Montefiore Medical Center in the Bronx, New York (NY), and The Miriam Hospital in Providence, Rhode Island (RI). Microbiome analyses on donor and patient fecal specimens were performed at the University of Minnesota in Minneapolis. The institutional review board at each center approved the study protocol (Supplement, available at www.annals.org). A data and safety monitoring board monitored the trial using halting rules for serious, unexpected, and related adverse safety outcomes, and all authors performed data analysis.
Study Population
The study population comprised adult outpatients who had 3 or more documented CDI recurrences (defined as ≥3 unformed stools over 24 hours for 2 consecutive days and either a positive stool test result for C difficile or pseudomembranes on colonoscopy) and who did not maintain cure after a course of tapered or pulsed vancomycin or were unable to taper or discontinue vancomycin (or an alternative antibiotic with activity against CDI) without recurrent diarrhea requiring anti-CDI treatment. All patients had completed at least 10 days of vancomycin therapy for the most recent CDI and continued therapy until 2 to 3 days before the procedure.
Major exclusion criteria included age 75 years or older; inflammatory bowel disease, irritable bowel syndrome (IBS), or chronic diarrheal disorder; any immunocompromised state or immunodeficiency; anaphylactic food allergy; previous FMT; untreated, in situ colorectal cancer; and inability to undergo colonoscopy.
Donor Identification and Screening
Patients were permitted to identify a donor or choose to be treated with stool from healthy volunteers who were recruited at each site. All prospective donors underwent a medical interview and physical examination and were excluded if they had a known communicable disease, features of the metabolic syndrome, a diarrheal disorder, an autoimmune or atopic disease, a tumor, a neurologic disorder, or chronic pain syndrome or if they had used antibiotics for any indication within 3 months. Potential donors also completed a modified AABB full-length donor history questionnaire, and those with risk factors for infectious agents were excluded (Supplement). Testing for HIV-1 and HIV-2 was performed within 2 weeks before donation for FMT. Other serologic and stool testing was performed within 1 month before FMT and included testing for hepatitis A, B, and C viruses; testing for Treponema pallidum; polymerase chain reaction (PCR) testing for detection of C difficile toxin; culture for enteric pathogens (Escherichia coli, Salmonella, Shigella, Yersinia, Campylobacter, Listeria monocytogenes, Vibrio parahaemolyticus, and V cholerae); testing for fecal Giardia and Cryptosporidium antigens; acid-fast stain for detection of Cyclospora and Isospora; ova and parasite testing; and enzyme immunoassay for detection of Rotavirus. Patients also had baseline testing for HIV-1 and HIV-2; hepatitis A, B, and C viruses; and T pallidum.
Randomization and Interventions
Donors took an osmotic laxative (magnesium hydroxide) the evening before and provided fresh stool the day of FMT. All donor specimens were transported on ice and processed within 6 hours of collection. Patients were given a standard bowel purge (sodium sulfate, potassium sulfate, and magnesium sulfate oral solution) the evening before the procedure. For patient convenience, sodium sulfate, potassium sulfate, and magnesium sulfate oral solution was substituted for the polyethylene glycol (PEG) bowel purge described in the study protocol. After initiating the preparation, patients were required to collect the first stool passed (for possible use in autologous FMT), transfer it to a clean container, and keep it either refrigerated or on ice. Within 1 hour before the scheduled FMT procedure, the nonblinded research assistant took possession of the stool specimens from both the donor and the patient. Patients were equally allocated to the donor and autologous FMT groups via block randomization by C difficile positivity at baseline, with stratification by study site. The protocol specified a “dose” of 100 g of stool diluted in 500 mL of nonbacteriostatic 0.9% normal saline immediately before the procedure, but the study relied on fresh stool, which has unpredictable weight and volume, and most provided specimens were less than 100 g. Because patients had discontinued use of vancomycin, had completed bowel lavage, and had been randomly assigned, we elected to use the lesser amount of fecal material. In those circumstances, available stool was weighed and suspended in a proportionately reduced volume of saline. A mean stool dose of 64 g (SD, 25 g; range, 20 to 100 g) was infused for donor FMT.
Colonoscopies were performed within the endoscopy units of the study centers. Endoscopic findings and the depth of insertion were recorded. The study physician administered 300 mL of the fecal suspension through the instrument’s working channel into the furthest point reached (terminal ileum or cecum). After FMT, patients were transferred to the recovery area and encouraged to retain the stool for at least 1 hour. If they were unable to do so, the time of the first postprocedure bowel movement was recorded.
Follow-up
Patients were instructed to contact the clinical team if they had recurrence of diarrhea and were provided with a thermometer for daily oral temperature readings and a diary card to record solicited AEs for 7 days and unsolicited AEs for 30 days after transplantation. They were contacted by telephone 2 and 7 days after FMT and then biweekly for 8 weeks and questioned about stool form and frequency. All patients were seen in the clinic for follow-up 2 and 8 weeks after treatment, where they were assessed for infectious and gastrointestinal symptoms and underwent physical examination. They submitted stool specimens at baseline and at each post-FMT visit for C difficile PCR testing and microbiome analysis. All patients were contacted by telephone by a study representative 6 months after the last treatment to record any serious AEs (SAEs), new medical conditions or diagnoses, or changes in medical conditions or medications since the last study contact.
End Points
The primary efficacy end point was the rate of clinical cure in the intention-to-treat (ITT) population 8 weeks after FMT or at the time of early withdrawal. Clinical cure was defined as lack of CDI recurrence with maintenance of resolution (that is, <3 unformed stools per day) for 8 weeks without requirement for further antibiotics (metronidazole, vancomycin, or fidaxomicin). Because nucleic acid testing does not distinguish colonized patients from those with symptomatic disease (15), it cannot test for cure (2, 16); therefore, patients meeting the definition of clinical cure were considered to be cured regardless of the results of follow-up PCR testing of stool for C difficile. Safety end points were evaluated by review of SAEs, AEs, new medical conditions or diagnoses, or changes in medical conditions at the 6-month follow-up.
Analysis of Fecal Microbiota
Fecal microbiota was analyzed from patients at least 5 days before and 2 and 8 weeks after FMT, as well as from donor samples. DNA extraction, 16S ribosomal RNA gene amplification, and sequencing were performed by the University of Minnesota Genomics Center. Processing details are described in the Supplement, and data are archived in the Sequence Read Archive at the National Center for Biotechnology Information under BioProject accession number SRP066964.
Taxonomic assignments were made against the Ribosomal Database Project using mothur software, version 1.34.0 (17). Alpha and beta diversity of bacterial communities were evaluated using the same software. Shannon indices and abundance-based coverage estimate parameters were calculated to assess alpha diversity. Differences in beta diversity and abundances of genera were performed using analysis of similarity and Kruskal–Wallis analysis, respectively (18).
Statistical Analysis
Fecal microbiota transplantation was predicted to reduce the incidence of relapse from 50% to 10% at the end of the study (4, 9). A sample size of 48 at the time of analysis was estimated to have greater than 80% power at a 2-sided α level of 4.7%. To account for dropouts, we planned to enroll 53 patients. All analyses were performed on the blinded treatment groups on an ITT basis.
Analyses were performed using Stata, version 12 (StataCorp), and SAS, version 9.4 (SAS Institute). A 2-sample t test, with adjustment for unequal variances applied when appropriate, was used to compare continuous outcome and other measures between treatment groups and sites. A generalized linear model for binary data was used to compare groups on dichotomous variables. Group, site, and the interaction of site and group were included in the model. The α level was set to 0.05 for statistical significance.
All AEs were summed across the entire diary period and compared between treatment groups by using 2 separate generalized linear mixed models for negative binomial–distributed count data. The first model compared raw counts, and the second model included the natural logarithm of the number of complete diary days as an offset to model the average daily rate of each dependent variable. Each model also included site as a random effect. The α level was set to 0.05 for all models.
Role of the Funding Source
This study was sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases, which had no role in the design of the study; collection, analysis, or interpretation of the data; writing, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Results
Patients
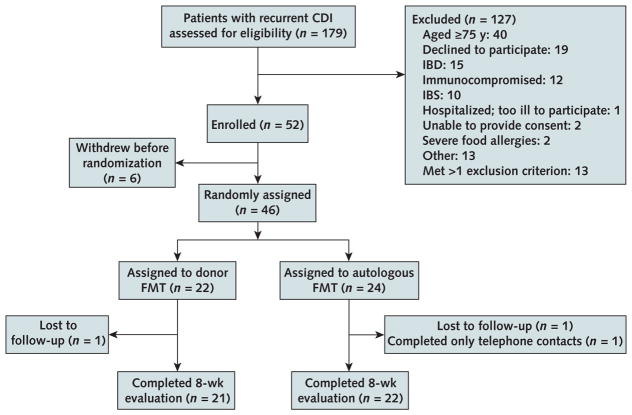
A total of 179 patients were evaluated, and 52 were enrolled (Figure 1). Of 62 potential donors, 49 were approved. For the blinded portion of the study, each patient had a unique donor. Patients were generally healthy while using vancomycin and were not acutely ill at the time of FMT. Only 3 were positive for C difficile by PCR at baseline (while using vancomycin). None had relapse during the 2 to 3 days without vancomycin before the procedure. Two patients in RI and 3 in NY withdrew before randomization. One NY patient had colon cancer discovered during FMT colonoscopy and was withdrawn. Forty-six patients were randomly assigned: 22 and 24 underwent donor and autologous FMT, respectively (Table 1). Forty-three patients completed the 8-week follow-up evaluation.
Figure 1.
Enrollment and outcomes.
Two patients at the Rhode Island site withdrew from the study before randomization. One reported that his CDI had resolved, and the other withdrew after the donor was unable to produce stool on the day of the planned FMT. Three patients in New York were determined to no longer have CDI after further evaluation by the investigator at that site and were withdrawn from the study before randomization. One patient in New York was found to have colon cancer (an exclusion criterion) at the time of the FMT colonoscopy and was withdrawn from the study. CDI = Clostridium difficile infection; FMT = fecal microbiota transplantation; IBD = inflammatory bowel disease; IBS = irritable bowel syndrome.
Table 1.
Baseline Characteristics of Enrolled Patients, by Group
| Characteristic | Donor FMT (n = 22) | Autologous FMT (n = 24) |
|---|---|---|
| Mean age (SD), y | 48 (16) | 55 (14) |
| Female, n (%) | 18 (82) | 19 (79) |
| Mean body mass index (SD), kg/m2 | 28 (8) | 27 (7) |
| Median Charlson Comorbidity Index score (range) | 1 (0–4) | 0 (0–3) |
| Mean duration of CDI since initial diagnosis (SD) [range], mo | 9 (9) [3–36] | 12 (12) [3–48] |
| Mean CDI recurrences (SD) [range], n | 4 (2) [3–9] | 5 (2) [2–10] |
| Mean duration of oral vancomycin therapy (SD) [range], wk* | 28 (36) [6–140] | 23 (30) [8–148] |
| Prior probiotic treatment, n (%) | 15 (68) | 18 (75) |
| Prior Lactobacillus GG use, n (%) | 5 (23) | 3 (13) |
| Prior Saccharomyces boulardii use, n (%) | 11 (50) | 13 (54) |
| Prior rifaximin use, n (%) | 3 (13) | 1 (4) |
| Prior fidaxomicin use, n (%) | 6 (27) | 8 (33) |
| Proton-pump inhibitor use, n (%) | 2 (9) | 2 (8) |
CDI = Clostridium difficile infection; FMT = fecal microbiota transplantation.
For all prior CDI treatment courses combined; does not necessarily represent continuous use.
Study Outcomes
In the ITT analysis, 20 of 22 patients (90.9% [95% CI, 69.2% to 97.8%]) in the donor FMT group achieved clinical cure versus 15 of 24 (62.5% [CI, 41.6% to 79.6%]) in the autologous FMT group. Donor FMT was statistically superior to autologous FMT (P = 0.042), but efficacy varied by site. In RI, the cure rate with donor FMT was 90.0% (CI, 51.8% to 98.7%) versus 42.9% (CI, 20.1% to 69.0%) with autologous FMT, whereas in NY, 91.7% (CI, 57.2% to 98.9%) of patients achieved clinical cure after donor FMT compared with 90.0% (CI, 51.8% to 98.7%) after autologous FMT (Figure 2). The interaction between sites was not statistically significant (P = 0.24), with an odds ratio of 9.8 (CI, 0.2 to 453.0) for treatment effects between sites.
Figure 2.
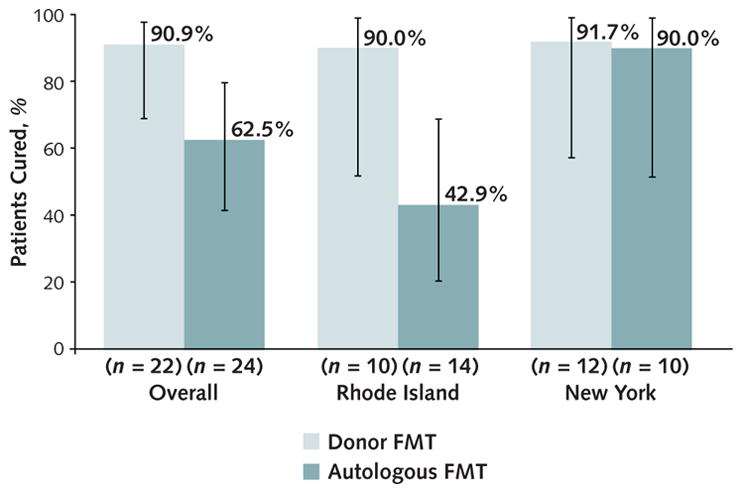
Rates of clinical cure in the intention-to-treat population, overall and by site.
Error bars represent 95% CIs. FMT = fecal microbiota transplantation.
Clinical failure occurred a mean of 10 days after the procedure (range, 1 to 62 days). Two patients did not maintain cure after donor FMT; only 20 g of donor stool was infused in one, and the other had received 60 g. One patient in the donor FMT group reported diarrhea at the 2-week visit and was subsequently lost to follow-up; the case was considered a clinical failure. One patient in the autologous FMT group was lost to follow-up after the 5-week telephone contact. She remained CDI-free at the last contact and per a subsequent primary care visit documented in the electronic medical record and was classified as cured. Another patient in the autologous FMT group who completed only telephone contacts was also classified as cured after she remained free of diarrhea through 8 weeks of follow-up. One of 2 patients who did not maintain cure after donor FMT was re-treated using a different donor and was subsequently cured.
All 9 patients who had recurrence after autologous FMT subsequently crossed over to treatment with donor FMT and were free of further CDI. Thus, the overall cure rate after a single donor FMT was 93.5% (29 of 31 patients). All cured patients remained free of late CDI recurrence between 8 and 24 weeks. Three cured patients had formed stools that were positive on PCR testing at 8 weeks; the others remained negative on PCR.
In a comparison of clinical features by site (Table 2), the duration of CDI and the mean number of recurrences were significantly higher in NY. Furthermore, more patients had been treated with fidaxomicin and proton-pump inhibitors in NY, whereas patients in RI reported greater Lactobacillus use before FMT. Several NY patients were treated with long courses of vancomycin before enrollment, although the duration reported was the cumulative weeks of therapy for all prior CDI episodes and did not necessarily represent continuous use.
Table 2.
Baseline Characteristics of Enrolled Patients, by Site
| Characteristic | Rhode Island (n = 24) | New York (n = 22) | P Value |
|---|---|---|---|
| Mean age (SD), y | 51 (15) | 52 (16) | 0.86 |
| Female, n (%) | 21 (77) | 19 (79) | 0.85 |
| Mean body mass index (SD), kg/m2 | 29 (7) | 26 (9) | 0.24 |
| Median Charlson Comorbidity Index score (range) | 0 (0–4) | 1 (0–3) | 0.22 |
| Mean duration of CDI since initial diagnosis (SD) [range], mo | 6 (2) [3–11] | 16 (14) [3–48] | 0.006 |
| Mean CDI recurrences (SD) [range], n | 4 (1) [2–6] | 5 (2) [3–10] | <0.001 |
| Mean duration of oral vancomycin therapy (SD) [range], wk* | 15 (7) [7–28] | 37 (45) [3–148] | 0.22 |
| Prior probiotic treatment, n (%) | 20 (83) | 13 (59) | 0.068 |
| Prior Lactobacillus GG use, n (%) | 7 (29) | 1 (5) | 0.028 |
| Prior Saccharomyces boulardii use, n (%) | 15 (63) | 9 (41) | 0.143 |
| Prior rifaximin use, n (%) | 1 (4) | 3 (14) | 0.26 |
| Prior fidaxomicin use, n (%) | 4 (17) | 10 (45) | 0.034 |
| Proton-pump inhibitor use, n (%) | 0 (0) | 4 (18) | 0.029 |
CDI = Clostridium difficile infection.
For all prior CDI treatment courses combined; does not necessarily represent continuous use.
Adverse Events
A complete description of AEs is included in the Supplement. Chills were reported more frequently after autologous than donor FMT (P = 0.053). Rates of other solicited AEs (fever, abdominal pain, bloating, nausea, vomiting, diarrhea, flatulence, anorexia, and constipation) did not differ significantly between groups. Four SAEs were reported, none were directly related to FMT or the colonoscopy. One patient was hospitalized for postpolypectomy gastrointestinal bleeding 4 months after donor FMT. Three other SAEs occurred in patients undergoing autologous FMT. Five patients reported new medical conditions or changes in established conditions at the 6-month follow-up, although none seemed to be related to FMT. Two patients had planned elective surgery and were treated with perioperative antibiotics; neither reported recurrence of CDI.
Microbiome Analyses
Before FMT, all patient samples showed marked dysbiosis with lower alpha diversity (Table 1 of the Supplement), more Gammaproteobacteria and Betaproteobacteria, and fewer Firmicutes and Bacteroidetes compared with donor samples (Figure 3). Donor FMT was associated with normalization of fecal microbiota and restoration of alpha diversity. Similar patterns were seen in microbiota from post-FMT samples after donor FMT that was performed as rescue after an initial failure. In contrast, dysbiosis persisted in autologous FMT patients who did not have relapse and was characterized by decreased Proteobacteria and a nonsignificant change in Bacteroidetes. Klebsiella, Enterobacter, Escherichia, Lactobacillus, and Veillonella were abundant in pre-FMT samples and were restricted in donors and donor FMT patients. Relative abundances of Bacteroides, Blautia, Faecalibacterium, and Roseburia were greater in donor fecal samples and in patients undergoing donor FMT.
Figure 3.
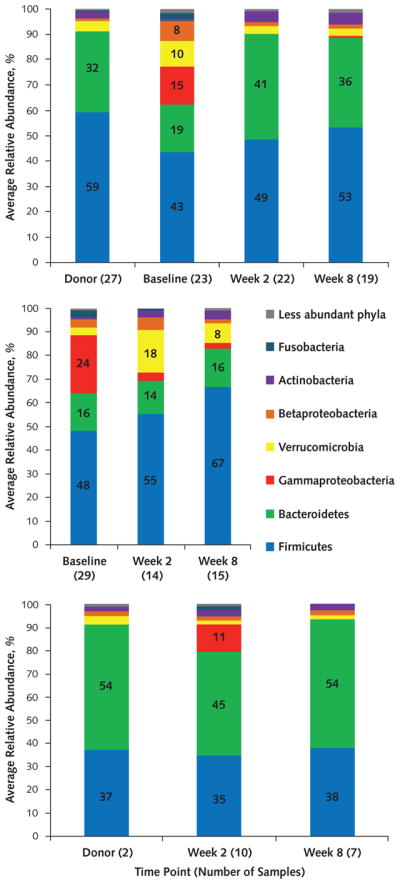
Distribution of phyla in samples from patients initially having donor FMT (top), those initially having autologous FMT (middle), and those having donor FMT after relapse (bottom) at both sites.
Less abundant phyla were present at a mean abundance <1.0% among all samples. FMT = fecal microbiota transplantation.
Fecal bacterial community composition of both donor and pre-FMT samples differed between sites. Samples from RI patients contained more Gammaproteobacteria and Betaproteobacteria (Figure 2 of the Supplement), whereas pre-FMT samples from NY patients commonly showed more Verrucomicrobia. There was a trend toward more Bacteroidetes in the NY samples but no difference in Bacteroidetes between patients with or without a history of fidaxomicin treatment. Fidaxomicin use correlated with more Verrucomicrobia and fewer Gammaproteobacteria before FMT (Figure 3 of the Supplement). After autologous FMT, NY patients had greater abundances of Clostridium species (Figure 1 of the Supplement).
Feces from the NY patient who underwent donor FMT and relapsed after the 2-week follow-up still had a high proportion of Gammaproteobacteria (12%) (Figure 4 [panel A] of the Supplement), despite an expansion of Bacteroidetes (27%) and increased alpha diversity (Shannon index, 3.81; abundance-based coverage estimate, 871). In contrast, the RI patient who had relapse at around 8 weeks after autologous FMT showed a community that taxonomically resembled that of donor samples through the end of the study (Figure 4 [panel B] of the Supplement); expansion of Bacteroidetes and Firmicutes was observed after autologous FMT, but dysbiosis was still apparent because alpha diversity did not recover.
Discussion
In this double-blind, randomized, controlled trial of FMT for treatment of recurrent CDI, donor FMT seemed safe and was more efficacious than autologous FMT. Overall, 91% of patients in the donor FMT group achieved clinical cure compared with 63% in the autologous FMT group, which is consistent with previously reported efficacy (10–12). Donor FMT restored normal microbial community structure, with reductions in Proteobacteria and Verrucomicrobia and increases in Bacteroidetes and Firmicutes. In contrast, microbial diversity did not improve after autologous FMT. In the absence of CDI recurrence, microbial community structure continued to evolve after autologous FMT, probably due to discontinuation of antibiotic use.
That many patients responded to autologous FMT is not unexpected, given that 35% to 65% of recurrent CDI is cured after a course of vancomycin (4, 5). However, cure rates with autologous FMT differed between study sites, prompting the question of why 90% of NY patients treated with their own stool were cured. Analysis of the cohorts at each site revealed clinical differences that may be significant. Patients in NY had CDI for a longer time, had more CDI recurrences, and received more courses of fidaxomicin than did RI patients. Many NY patients had waited for long periods before consultation and had been receiving extended courses of vancomycin (up to 148 weeks) and other therapies (such as fidaxomicin) before entering the study. Some of these patients could have been cured before enrollment. Microbiome analyses showed differences in preprocedural composition of fecal samples between sites. Autologous FMT patients at the NY site had greater abundances of Clostridia, raising the possibility of emergence of microbial community assemblages inhibitory to C difficile via competitive niche exclusion or possibly by emergence of nontoxigenic organisms (19). Alternatively, the infectious burden of C difficile might have decreased below a critical level due to a longer course of antibiotic suppression. It is also possible that some of the patients enrolled in this trial were colonized with C difficile and ongoing diarrheal symptoms were related to postinfection IBS. Piche and colleagues previously showed that 35% of patients developed new IBS symptoms shortly after resolution of CDI (20). Patients with IBS may report improvement in symptoms after a course of antibiotics (21), making it difficult to distinguish diarrheal symptoms related to IBS from those of CDI; some patients who were “cured” by autologous FMT may have been in this category. This is likely a common real-world scenario given the limits of current nucleic acid testing, which does not distinguish active CDI from asymptomatic carriage (15).
The main strengths of this study are its blinding, the multicenter design using autologous FMT as a “placebo,” and the microbiome analyses. The widespread adoption of FMT was based on efficacy shown in case series and open-label trials. Conducting a blinded, controlled trial using fresh donor stool was challenging but was necessary to investigate efficacy of this therapy. At the end of the trial, all patients cured with autologous FMT speculated that they had received donor stool, suggesting an adequate blind. Microbiome analyses provided important mechanistic data. Shifts in bacterial community after FMT for patients who underwent donor FMT are similar to those reported previously (22, 23).
This study has several limitations. First, no data were collected on the severity of prior CDI episodes or on baseline antibody titers. Second, persons aged 75 years or older were excluded, and the mean age of the enrolled patients was much younger; hence, the results may not be representative of most patients with CDI. This age restriction was required by the U.S. Food and Drug Administration in order for us to perform this study using FMT as an Investigational New Drug. Age is a strong risk factor for CDI and recurrence (24), and FMT has been shown to be safe and effective in elderly patients (25). Future trials should include this population as well as immunocompromised patients, another excluded group that may benefit from FMT (26).
Third, we did not meet the target of 48 randomly assigned patients as planned. We terminated enrollment after 28 months and randomization of 46 patients because of growing data on the efficacy of FMT (10, 11) and its increased availability, which influenced patients’ willingness to be enrolled as well as the ethics of conducting a placebo-controlled study.
Fourth, the doses of stool that were administered to patients differed. Only 2 patients treated with donor FMT had relapse, one received 20 g and the other received 60 g. Three other patients were cured with donor stool doses of 10 to 20 g, suggesting that efficacy was not clearly related to the amount of stool administered, although failure after donor FMT was too infrequent to determine whether dose was a factor.
Fifth, PCR testing of stool was used to diagnose CDI. Although this is the currently recommended method (16), a recent study suggested that reliance on highly sensitive molecular testing may result in overdiagnosis and overtreatment (27). Nucleic acid testing does not distinguish colonized patients from those with symptomatic disease (15), and stool may remain positive on PCR up to 30 days after successful treatment (28). We recently published data showing that 25% of patients referred for FMT did not have CDI but rather an alternative diagnosis (29). Clearly, not all patients with “recurrent CDI” need additional courses of expensive antibiotics or FMT. Efforts to refine the imperfect CDI diagnostic algorithm and to develop alternative assays to complement nucleic acid testing would be beneficial and would prevent unnecessary antibiotic treatment and FMT. Finally, although we documented no SAEs related to FMT, the study was not powered to detect rare safety outcomes, and a larger trial or national patient registry would help answer questions about the safety of FMT.
In conclusion, FMT using fresh donor stool administered via colonoscopy after a course of vancomycin was effective at preventing further CDI episodes in patients with multiply recurrent infection. Differences in efficacy between sites suggest that some patients with lower risk for CDI recurrence may not benefit from FMT. Further research may help determine the best candidates for FMT.
Supplementary Material
Acknowledgments
The authors thank all of the patients who participated in this study; Drs. Christina Surawicz and Pierre Gholam, who served on the data and safety monitoring board; the staff of the Women’s Medicine Collaborative; and Beth Hott for her help with the manuscript submission.
Grant Support: Drs. Kelly and Brandt received funding from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (1R21DK0939839). Drs. Khoruts and Sadowsky received grant support from the National Institutes of Health (R21AI114722-01).
Footnotes
Reproducible Research Statement: Study protocol: See the Supplement. Statistical code and data set: The authors are willing to share the statistical code used to generate results and the data set from which the results were derived with the public after written agreement. Requests should be directed to Dr. Kelly (, colleen_r_kelly@brown.edu).
Current author addresses and author contributions are available at www.annals.org.
Disclosures: Dr. Kelly reports a grant from Assembly Biosciences and other support from Seres Health outside the submitted work. Dr. Khoruts reports a grant from CIPAC outside the submitted work and a patent pending for compositions and methods for transplantation of colon microbiota. Dr. Sadowsky reports grants and personal fees from CIPAC during the conduct of the study and outside the submitted work and patents with royalties paid to CIPAC. Dr. Brandt reports a grant from the National Institutes of Health during the conduct of the study and nonfinancial support from OpenBiome and personal fees from CIPAC/Crestovo outside the submitted work. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M16-0271.
Author Contributions: Conception and design: C.R. Kelly, M.J. Sadowsky, J.T. Machan, L.J. Brandt.
Analysis and interpretation of the data: C.R. Kelly, A. Khoruts, C. Staley, M.J. Sadowsky, S.E. Reinert, J.T. Machan, L.J. Brandt.
Drafting of the article: C.R. Kelly, A. Khoruts, C. Staley, M.J. Sadowsky, S.E. Reinert, J.T. Machan, L.J. Brandt.
Critical revision of the article for important intellectual content: C.R. Kelly, A. Khoruts, C. Staley, M.J. Sadowsky, J.T. Machan, L.J. Brandt.
Final approval of the article: C.R. Kelly, A. Khoruts, C. Staley, M.J. Sadowsky, M. Abd, M. Alani, B. Bakow, P. Curran, J. McKenney, A. Tisch, S.E. Reinert, J.T. Machan, L.J. Brandt.
Provision of study materials or patients: M.J. Sadowsky, M. Abd, L.J. Brandt.
Statistical expertise: S.E. Reinert, J.T. Machan.
Obtaining of funding: J. McKenney.
Administrative, technical, or logistic support: M. Abd, M. Alani, B. Bakow.
Collection and assembly of data: C.R. Kelly, C. Staley, M. Abd, M. Alani, B. Bakow, P. Curran, J. McKenney, A. Tisch, L.J. Brandt.
References
- 1.Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825–34. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Society for Healthcare Epidemiology of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–55. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 3.Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478–98. doi: 10.1038/ajg.2013.4. [DOI] [PubMed] [Google Scholar]
- 4.McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97:1769–75. doi: 10.1111/j.1572-0241.2002.05839.x. [DOI] [PubMed] [Google Scholar]
- 5.McFarland LV, Surawicz CM, Rubin M, Fekety R, Elmer GW, Greenberg RN. Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect Control Hosp Epidemiol. 1999;20:43–50. doi: 10.1086/501553. [DOI] [PubMed] [Google Scholar]
- 6.Chang JY, Antonopoulos DA, Kalra A, Tonelli A, Khalife WT, Schmidt TM, et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197:435–8. doi: 10.1086/525047. [DOI] [PubMed] [Google Scholar]
- 7.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53:994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 8.Guo B, Harstall C, Louie T, Veldhuyzen van Zanten S, Dieleman LA. Systematic review: faecal transplantation for the treatment of Clostridium difficile-associated disease. Aliment Pharmacol Ther. 2012;35:865–75. doi: 10.1111/j.1365-2036.2012.05033.x. [DOI] [PubMed] [Google Scholar]
- 9.Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108:500–8. doi: 10.1038/ajg.2013.59. [DOI] [PubMed] [Google Scholar]
- 10.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–15. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 11.Cammarota G, Masucci L, Ianiro G, Bibbò S, Dinoi G, Costamagna G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015;41:835–43. doi: 10.1111/apt.13144. [DOI] [PubMed] [Google Scholar]
- 12.Lee CH, Steiner T, Petrof EO, Smieja M, Roscoe D, Nematallah A, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: a randomized clinical trial. JAMA. 2016;315:142–9. doi: 10.1001/jama.2015.18098. [DOI] [PubMed] [Google Scholar]
- 13.Baxter M, Ahmad T, Colville A, Sheridan R. Fatal aspiration pneumonia as a complication of fecal microbiota transplant [Letter] Clin Infect Dis. 2015;61:136–7. doi: 10.1093/cid/civ247. [DOI] [PubMed] [Google Scholar]
- 14.Zipursky JS, Sidorsky TI, Freedman CA, Sidorsky MN, Kirkland KB. Patient attitudes toward the use of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection. Clin Infect Dis. 2012;55:1652–8. doi: 10.1093/cid/cis809. [DOI] [PubMed] [Google Scholar]
- 15.Koo HL, Van JN, Zhao M, Ye X, Revell PA, Jiang ZD, et al. Real-time polymerase chain reaction detection of asymptomatic Clostridium difficile colonization and rising C. difficile-associated disease rates. Infect Control Hosp Epidemiol. 2014;35:667–73. doi: 10.1086/676433. [DOI] [PubMed] [Google Scholar]
- 16.Wilcox MH, Planche T, Fang FC, Gilligan P. What is the current role of algorithmic approaches for diagnosis of Clostridium difficile infection? J Clin Microbiol. 2010;48:4347–53. doi: 10.1128/JCM.02028-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–41. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acar EF, Sun L. A generalized Kruskal-Wallis test incorporating group uncertainty with application to genetic association studies. Biometrics. 2013;69:427–35. doi: 10.1111/biom.12006. [DOI] [PubMed] [Google Scholar]
- 19.Gerding DN, Meyer T, Lee C, Cohen SH, Murthy UK, Poirier A, et al. Administration of spores of nontoxigenic Clostridium difficile strain M3 for prevention of recurrent C. difficile infection: a randomized clinical trial. JAMA. 2015;313:1719–27. doi: 10.1001/jama.2015.3725. [DOI] [PubMed] [Google Scholar]
- 20.Piche T, Vanbiervliet G, Pipau FG, Dainese R, Hébuterne X, Rampal P, et al. Low risk of irritable bowel syndrome after Clostridium difficile infection. Can J Gastroenterol. 2007;21:727–31. doi: 10.1155/2007/262478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, et al. TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton MJ, Weingarden AR, Unno T, Khoruts A, Sadowsky MJ. High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria. Gut Microbes. 2013;4:125–35. doi: 10.4161/gmic.23571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shankar V, Hamilton MJ, Khoruts A, Kilburn A, Unno T, Paliy O, et al. Species and genus level resolution analysis of gut microbiota in Clostridium difficile patients following fecal microbiota transplantation. Microbiome. 2014;2:13. doi: 10.1186/2049-2618-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandt LJ, Aroniadis OC, Mellow M, Kanatzar A, Kelly C, Park T, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:1079–87. doi: 10.1038/ajg.2012.60. [DOI] [PubMed] [Google Scholar]
- 25.Agrawal M, Aroniadis OC, Brandt LJ, Kelly C, Freeman S, Surawicz C, et al. The long-term efficacy and safety of fecal microbiota transplant for recurrent, severe, and complicated Clostridium difficile infection in 146 elderly individuals. J Clin Gastroenterol. 2016;50:403–7. doi: 10.1097/MCG.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 26.Kelly CR, Ihunnah C, Fischer M, Khoruts A, Surawicz C, Afzali A, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109:1065–71. doi: 10.1038/ajg.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polage CR, Gyorke CE, Kennedy MA, Leslie JL, Chin DL, Wang S, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med. 2015;175:1792–801. doi: 10.1001/jamainternmed.2015.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Surawicz CM, McFarland LV, Greenberg RN, Rubin M, Fekety R, Mulligan ME, et al. The search for a better treatment for recurrent Clostridium difficile disease: use of high-dose vancomycin combined with Saccharomyces boulardii. Clin Infect Dis. 2000;31:1012–7. doi: 10.1086/318130. [DOI] [PubMed] [Google Scholar]
- 29.Jackson M, Olefson S, Machan JT, Kelly CR. A high rate of alternative diagnoses in patients referred for presumed Clostridium difficile infection. J Clin Gastroenterol. 2015 doi: 10.1097/MCG.0000000000000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.