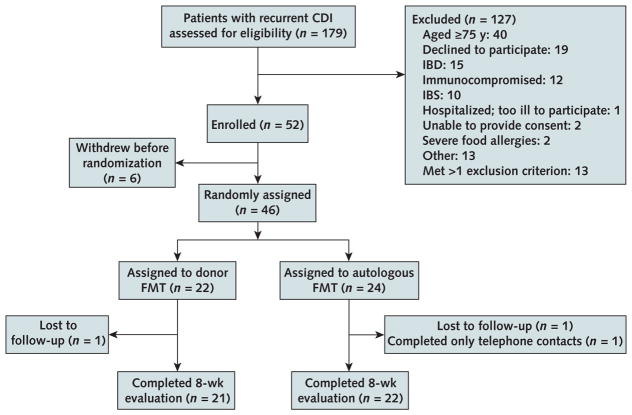
Figure 1.
Enrollment and outcomes.
Two patients at the Rhode Island site withdrew from the study before randomization. One reported that his CDI had resolved, and the other withdrew after the donor was unable to produce stool on the day of the planned FMT. Three patients in New York were determined to no longer have CDI after further evaluation by the investigator at that site and were withdrawn from the study before randomization. One patient in New York was found to have colon cancer (an exclusion criterion) at the time of the FMT colonoscopy and was withdrawn from the study. CDI = Clostridium difficile infection; FMT = fecal microbiota transplantation; IBD = inflammatory bowel disease; IBS = irritable bowel syndrome.