Table 2.
Biological Activity of Ureidopropanamides Derived from Modification of Compound 2.
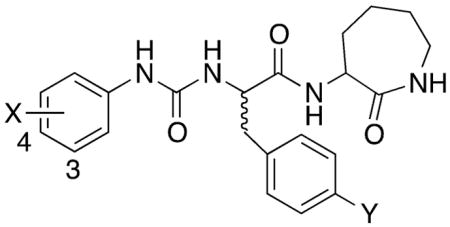
| |||||||
|---|---|---|---|---|---|---|---|
| Ca+2 mobilization | Ca+2 mobilization | MTT | |||||
| FPR2-HL60 | FPR1-HL60 | FPR2-HL60 | FPR1-HL60 | ||||
| Compd. | X | Y | EC50, μM (efficacy, %)[a] | EC50, μM (efficacy, %)[a] | IC50, μM (max. inhibition, %)[a] | IC50, μM (max. inhibition, %)[a] | EC50, μM[a] |
| (S)-2 | 4-Br | H | 0.004 ± 0.002 (115)[b] | 0.3 ± 0.08 (135) [b] | N.T.b | N.T. | N.T. |
| (2R)-42 | 4-OH | H | N.A.c | N.A. | N.A. | N.A. | >100 |
| (2R)-43 | 3-OH | H | N.A. | 12.5 ± 2.5 (90) | N.A. | N.A. | >100 |
| (2S)-44 | 4-OH | H | N.A. | 11.0 ± 3.6 (65) | N.A. | N.A. | >100 |
| (2S)-45 | 3-OH | H | N.A. | 0.55 ± 0.13 (90) | N.A. | 1.2 ± 0.4 (90) | >100 |
| (2R)-46 | 4-Br | OH | 0.35 ± 0.12 (140) | 0.82 ± 0.37 (110) | 25.3 ± 7.2 (100) | 0.56 ± 0.17 (85) | >100 |
| (2S)-47 | 4-Br | OH | 0.78 ± 0.23 (90) | 0.23 ± 0.05 (125) | 2.1 ± 0.6 (100) | 0.076 ± 0.014 (95) | >100 |
data are the mean of three indipendent experiments; N.T.= not tested; N.A.= not active;
data taken from ref. [28].
