Abstract
Maternal glycemia is a key determinant of birth weight, but recent large-scale genome-wide association studies demonstrated an important contribution of fetal genetics. It is not known whether fetal genotype modifies the impact of maternal glycemia or whether it acts through insulin-mediated growth. We tested the effects of maternal fasting plasma glucose (FPG) and a fetal genetic score for birth weight on birth weight and fetal insulin in 2,051 European mother-child pairs from the Exeter Family Study of Childhood Health (EFSOCH) and the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study. The fetal genetic score influenced birth weight independently of maternal FPG and impacted growth at all levels of maternal glycemia. For mothers with FPG in the top tertile, the frequency of large for gestational age (birth weight ≥90th centile) was 31.1% for offspring with the highest tertile genetic score and only 14.0% for those with the lowest tertile genetic score. Unlike maternal glucose, the fetal genetic score was not associated with cord insulin or C-peptide. Similar results were seen for HAPO participants of non-European ancestry (n = 2,842 pairs). This work demonstrates that for any level of maternal FPG, fetal genetics has a major impact on fetal growth and acts predominantly through independent mechanisms.
Introduction
Maternal glycemia is a major determinant of fetal growth, with there being a strong, continuous association between maternal fasting glucose levels and offspring birth weight (1). In clinical practice, women identified as having gestational diabetes mellitus are at a high risk of having a baby large for gestational age (LGA) (birth weight ≥90th centile) if their hyperglycemia is untreated. Insulin is a potent growth factor (2), and the association between maternal hyperglycemia and higher birth weight is a result of fetal hyperinsulinemia in response to maternal hyperglycemia (3). However, maternal fasting glycemia explains only 2–13% of the variance in birth weight (4,5), and the majority of LGA babies are not born to mothers with diabetes (6).
Recent genome-wide association study (GWAS) data have indicated an important role for common maternal and fetal genetic variation in birth weight (7,8). Notably, Horikoshi et al. (8) identified robust associations of single nucleotide polymorphisms (SNPs) with birth weight at 60 individual loci from the fetal genome, some of which were known to be associated with glycemic traits in adults. The effects of the 60 loci could be mediated in part by the maternally inherited component of the fetal genotype and its influence on the intrauterine environment. However, analysis of available data suggested that the majority of these SNPs had direct fetal effects (8). The relative impact that these common fetal polymorphisms have on growth in the presence of maternal hyperglycemia is not known.
We generated a fetal genetic score for birth weight using the recently identified SNPs associated with birth weight (8) and analyzed its associations with offspring birth weight at varying levels of maternal fasting glucose in mother-offspring pairs. We tested the hypotheses 1) that the fetal genetic score and maternal fasting glucose level would interact to influence birth weight and 2) that both maternal fasting glucose and fetal genetic score would be associated with measures of fetal insulin from cord blood.
Research Design and Methods
Study Population
Women with singleton pregnancies and without pre-existing diabetes from the Exeter Family Study of Childhood Health (EFSOCH) (9) and the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study (1) and their offspring were included (Table 1). In EFSOCH, all women and their partners were of self-reported European ancestry (n = 701 mother-offspring pairs), and in HAPO, mother-offspring pairs were divided into separate analysis groups according to European (n = 1,350), Thai (n = 1,168), Afro-Caribbean (n = 1,072), and Mexican American (n = 602) ancestry (Supplementary Table 1). The study samples analyzed in the current study were independent of the GWAS in which the birth weight loci were identified (8). As the genetic variants were discovered in a sample that was mainly European and effect allele frequencies were not strongly correlated in all cases (see Supplementary Table 2), the main analyses for this article are for participants of European ancestry, and results for participants of non-European ancestry are presented in the online Supplementary Data.
Table 1.
Characteristics of mothers and offspring of European ancestry with maternal FPG value, genetic data, and offspring birth weight in EFSOCH and HAPO
| EFSOCH | HAPO | |
|---|---|---|
| Total mothers, n | 701 | 1,350 |
| Age in years, mean (SD) | 30.5 (5.2) | 31.2 (5.3) |
| Primiparous, n (%) | 298 (42.5) | 776 (57.5) |
| Prepregnancy BMI (kg/m2), mean (SD) | 24.0 (4.3) | 24.5 (5.0) |
| Smoker, n (%) | 94 (13.9)* | 185 (13.7) |
| Total offspring, n | 701 | 1,350 |
| Gestational age at delivery in weeks, mean (SD) | 40 (1) | 40 (1) |
| Female offspring, n (%) | 339 (48.4) | 676 (50.1) |
| Offspring birth weight (g) corrected for sex and gestational age, mean (SD) | 3,490 (432) | 3,423 (455) |
| Corrected birth weight cutoff (g) for LGA (≥90th centile) | 4,064 | 3,948 |
*Smoking data are for 94/675 mothers in EFSOCH.
Sample Acquisition
Fasting plasma glucose (FPG) in mmol/L was measured at approximately 28 weeks’ gestation in EFSOCH and HAPO participants. Babies were weighed following delivery using clinically validated scales. Umbilical cord blood was collected at delivery for offspring DNA extraction and measurement of insulin levels (in EFSOCH offspring) or C-peptide levels (in HAPO offspring).
Genotyping of EFSOCH Mothers and Babies
Genotyping of the whole EFSOCH sample (2,768 mothers, fathers, and offspring) was performed using the Illumina HumanCoreExome array. Included samples were of European ancestry (assessed using flashPCA [10]), had genotype call rates >98%, and had phenotypic sex and kinship validated using genotype data (the latter assessed using KING software [11]). Included genotyped SNPs had call rates >95%, Hardy-Weinberg P > 1 × 10−6, and minor allele frequency >1%. Samples were imputed to the Haplotype Reference Consortium (HRC) version r1.1 reference panel (Michigan Imputation Server), and SNPs with imputation quality score >0.4 and minor allele frequency >1% were included. Two (rs11096402 and rs139975827) of the 60 birth weight–associated SNPs (8) were unavailable because of poor imputation quality.
Genotyping of HAPO Mothers and Babies
Genotyping of HAPO samples was performed using Illumina genome-wide arrays at the Broad Institute (Cambridge, MA) or Johns Hopkins Center for Inherited Disease Research (Baltimore, MD). Quality control of genotype data was performed as described previously (12–14). Genotypes were imputed using SHAPEIT v.2 and IMPUTE2 v.2.3.0 with 1000 Genomes Phase 3 data. Details of genotyping platforms, genotype-calling algorithms, quality control and imputation procedures, and population substructure estimates were previously reported (12–14). One (rs11096402) of the 60 birth weight–associated SNPs was unavailable because of poor imputation quality.
Generating a Fetal Genetic Score for Birth Weight
A weighted fetal genetic score for birth weight was generated to take into account that some SNPs have a greater effect on birth weight than others. It was calculated by weighting the number of alleles by effect sizes reported in the original GWAS (8) (Supplementary Table 3) and rescaling to reflect the total number of SNPs used (Supplementary Fig. 1).
Statistical Analyses
The “corrected birth weight” variable was prepared by saving residuals from a linear regression analysis of birth weight (g) against sex and gestational age within EFSOCH or HAPO (including participants without genetic data). LGA was defined as a corrected birth weight ≥90th centile in each study. Linear regression was used to analyze associations between corrected birth weight or cord insulin (EFSOCH) or C-peptide (HAPO) and maternal FPG or the fetal genetic score for birth weight, with the latter two variables both as continuous variables and as ascending tertiles. Statistical interaction between the tertiles was analyzed using likelihood-ratio testing. The number of LGA babies was compared across the combined tertiles using logistic regression. A sensitivity analysis was performed for offspring of women with an FPG ≥5.1 mmol/L (the threshold recommended by the World Health Organization [WHO] for gestational diabetes mellitus diagnosis). Data from EFSOCH and HAPO Europeans were combined using inverse variance meta-analysis and showed minimal heterogeneity between the two study samples (I2 < 66.1%, all P > 0.09). All analyses were performed using Stata 14 (StataCorp LP, College Station, TX) or R version 3.3.1.
Ethics approval was obtained from the North and East Devon Local Research Ethics Committee (EFSOCH) and from the Northwestern University Office for the Protection of Research Subjects (HAPO).
Results
Fetal Genetic Score for Birth Weight Influences Birth Weight Independently of Maternal FPG
Basic characteristics of mothers and their offspring are provided in Table 1 and Supplementary Table 1. Maternal FPG and the fetal genetic score for birth weight were positively associated with corrected birth weight (all P < 0.001; Supplementary Table 4). Maternal FPG was not associated with the fetal genetic score for birth weight (β coefficient 0.002 mmol/L, mean adjusted R2 < 0.01, P = 0.25), indicating that they have separate, independent effects. Similar patterns of association between fetal genetic score and corrected birth weight were seen in participants of non-European ancestry, although there was a tendency toward smaller effect estimates: e.g., in the Afro-Caribbean and Thai samples the 95% CI did not include the European estimates (Supplementary Tables 5–7).
Fetal Genetic Score for Birth Weight and Maternal FPG Have an Additive Effect on Birth Weight
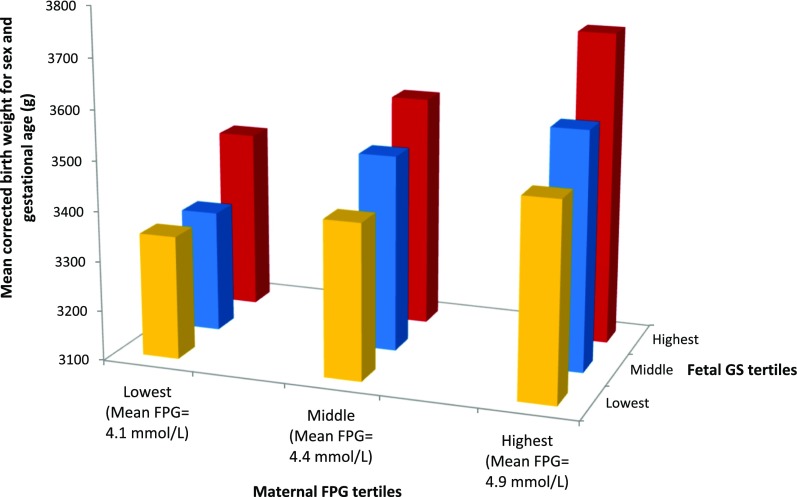
In each maternal FPG tertile, corrected birth weight was positively associated with fetal genetic score tertile in participants of European ancestry. Similarly, corrected birth weight was positively associated with maternal FPG tertile in each fetal genetic score tertile (Supplementary Table 8). There was no statistical interaction between maternal FPG and fetal genetic score tertiles (P = 0.92, likelihood-ratio test). Combining the maternal FPG and fetal genetic score tertiles resulted in an additive effect on birth weight (Fig. 1). Similar patterns of association were seen in participants of non-European ancestry (Supplementary Tables 9–11).
Figure 1.
The effect of combined maternal FPG and fetal genetic score for birth weight tertiles on birth weight. Bar chart showing mean birth weight (g) corrected for sex and gestational age across combined maternal FPG and fetal genetic score for birth weight tertiles for 2,051 offspring of European ancestry. Mean birth weight for all offspring of European ancestry was 3,448 ± 10 g. The lowest tertiles of the fetal genetic score for birth weight are shown in yellow, the middle tertiles in blue, and the highest tertiles in red. GS, genetic score for birth weight.
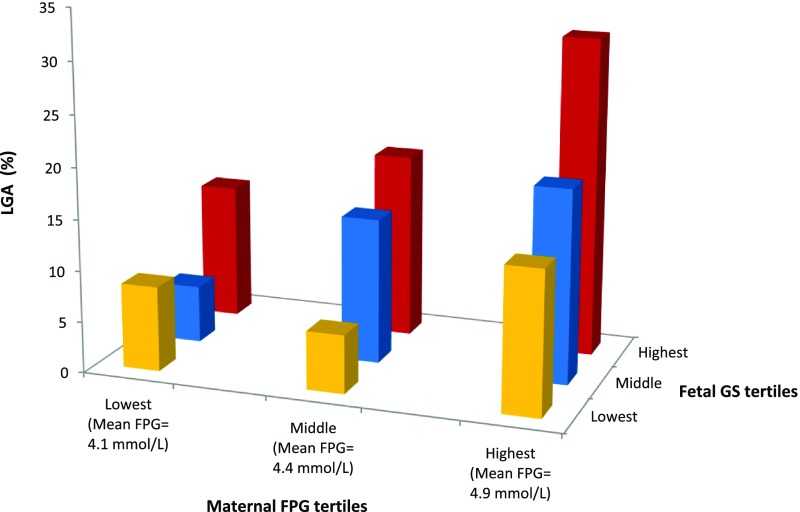
Fetal Genetic Score for Birth Weight Was Associated With LGA Frequency in the Middle and Highest Tertiles of Maternal FPG
There was a marked impact of fetal genotype on LGA; in the highest FPG tertile, 78 of 251 (31.1%) offspring were LGA when the fetus was in the highest fetal genetic score tertile and 31 of 221 (14.0%) were LGA when the fetus was in the lowest fetal genetic score tertile (odds ratio 2.76 [95% CI 1.74–4.40], P < 0.001; Supplementary Table 12 and Fig. 2). This result was consistent for those in the middle maternal FPG tertile (Supplementary Table 12). For non-European ancestry participants, effects were in the same direction, with wider CIs (Supplementary Tables 13–15).
Figure 2.
The effect of combined maternal FPG and fetal genetic score for birth weight tertiles on LGA prevalence. Bar chart showing the prevalence of LGA (%) across combined maternal FPG and fetal genetic score for birth weight tertiles in participants of European ancestry. The lowest tertiles of the fetal genetic score for birth weight are shown in yellow, the middle tertiles in blue, and the highest tertiles in red. GS, genetic score for birth weight.
Fetal Genetic Score for Birth Weight Was Associated With Birth Weight in Mothers Meeting the WHO FPG Criteria for Gestational Diabetes Mellitus
Corrected birth weight for offspring born to women who met the WHO criteria for gestational diabetes mellitus (FPG ≥5.1 mmol/L) increased with fetal genetic score tertile (P for linear trend <0.001). In addition, LGA frequency in the highest fetal genetic score tertile was 42.6% and the odds ratio for LGA in the highest versus the lowest tertile was 2.75 (Supplementary Table 16). The effect of fetal genetic score on LGA in participants of non-European ancestry with FPG ≥5.1 mmol/L was less clear (Supplementary Table 17).
Fetal Genetic Score for Birth Weight Does Not Influence the Fetal Insulin Response
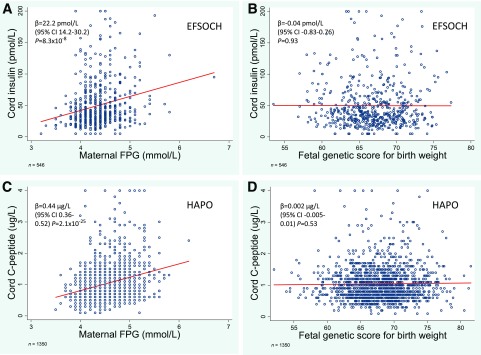
There was strong evidence of association between maternal FPG and cord insulin (EFSOCH) and cord C-peptide (HAPO) (P < 0.001 for both; Fig. 3A and C). However, neither cord insulin nor cord C-peptide was associated with the fetal genetic score (P = 0.93 and P = 0.53, respectively; Fig. 3B and D). Similar results were seen in non-European participants (Supplementary Fig. 2).
Figure 3.
The associations for maternal FPG and fetal genetic score for birth weight with fetal insulin levels. A and B: Scatter plots with linear regression lines (in red) showing associations for maternal FPG (A) and fetal genetic score for birth weight (B) with cord insulin level at birth in EFSOCH (n = 546). C and D: Scatter plots with linear regression lines (in red) showing associations for maternal FPG (C) and fetal genetic score for birth weight (D) with cord C-peptide in HAPO (n = 1,350). Cord insulin levels ≤5 pmol/L are truncated at 5 pmol/L and levels ≥200 pmol/L are truncated at 200 pmol/L. Cord C-peptide levels ≥4 µg/L are truncated at 4 µg/L.
Discussion
We have shown strong evidence of an effect of fetal genotype on birth weight, independent of maternal FPG, in 2,051 women of European ancestry and their offspring. Even though maternal FPG explained approximately twice the variance in birth weight as the fetal genetic score for birth weight, they had strikingly similar effects and, when combined, the effects were additive. The impact of this was such that in mothers with the highest FPG, the frequency of LGA in offspring in the highest fetal genetic score tertile (31.1%) was more than double that in offspring in the lowest fetal genetic score tertile (14.0%). Furthermore, in mothers with gestational diabetes mellitus (FPG ≥5.1 mmol/L), the equivalent percentages were 42.6% and 21.3% for the highest and lowest fetal genetic score tertiles, respectively. Thus, fetal genetics makes an important contribution to variation in fetal growth, even among cases of LGA that have traditionally been attributed to the intrauterine environment.
Maternal FPG influences fetal growth mainly by stimulating fetal insulin secretion (3) and was associated with cord insulin and C-peptide as expected. Conversely, the fetal genetic score for birth weight was not associated with cord insulin or C-peptide, indicating that the collective mechanisms of action of the SNPs in the fetal genetic score are largely independent of fetal insulin secretion. Gestational diabetes mellitus is associated with increased insulin-mediated growth and fetal adiposity (15), and excessive insulin secretion in utero has been shown to be a predictor of later obesity and impaired glucose tolerance (16). As there is an inverse relationship between loci associated with birth weight and cardiometabolic disease (8), it is possible that fetal genotype could predominantly contribute to variation in “normal” fetal growth without the same implications for later-life metabolic dysfunction. However, the fetal genetic score for birth weight is associated with newborn skin-fold thickness (Supplementary Table 18), which highlights the need for further investigation, including the consideration of the effects of these genetic variants on visceral fat, which may have different long-term implications (17).
At present, prediction of birth weight prior to term gestation is difficult, and previous studies modeling clinical risk factors, serum biomarkers, and fetal biometry measured by ultrasound to predict small for gestational age (birth weight <10th centile) and LGA achieved limited diagnostic performance (18,19). Our preliminary analyses (unpublished data) suggest the fetal genetic score for birth weight has predictive ability similar to maternal fasting glucose. Progress is being made in whole-genome sequencing of cell-free fetal DNA (20,21), and techniques to detect multiple SNPs associated with a complex trait such a birth weight may be established in the future. However, further studies are needed to optimize clinical prediction of birth weight and to assess the extent to which this approach has clinical utility.
A limitation of this study is that our main analyses considered the effect of a fetal genetic score for birth weight derived from a population of European ancestry, and this type of score may not be directly applicable to other populations. Although our additional analyses of non-European samples showed broadly similar trends, they were likely underpowered. A possible reason for the smaller observed effects of the fetal genetic score on birth weight in these samples was that interpopulation differences in linkage disequilibrium structure reduced the ability of the genetic score to capture the underlying causal genetic variation relative to the European sample. Further studies in large samples will be necessary to investigate potential interpopulation differences.
In conclusion, a fetal genetic score for birth weight influences fetal growth at different levels of maternal FPG. Its overall effect is independent of maternal glycemia and likely reflects multiple mechanisms, but it does not predominantly act through stimulating fetal insulin secretion.
Supplementary Material
Article Information
Acknowledgments. We acknowledge the work of the EFSOCH and HAPO investigators, whose names can be viewed in their original publications (1,9). We acknowledge the role of all professionals and families who contributed to EFSOCH and HAPO.
Funding. A.E.H. was an Academic Foundation Year 2 Doctor funded by the National Institute for Health Research (NIHR). R.N.B. and R.M.F. are funded by the Wellcome Trust and Royal Society, grant 104150/Z/14/Z. A.T.H. is a Wellcome Trust Senior Investigator and an NIHR Senior Investigator. EFSOCH was supported by South West National Health Service (NHS) Research and Development, Exeter NHS Research and Development, the Darlington Trust, and the Peninsula NIHR Clinical Research Facility at the University of Exeter. Genotyping of the EFSOCH study samples was funded by the Wellcome Trust and Royal Society, grant 104150/Z/14/Z. HAPO was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Diabetes and Digestive and Kidney Diseases, the National Center for Research Resources, and the American Diabetes Association.
The opinions given in this article do not necessarily represent those of NIHR, the NHS, or the U.K. Department of Health and Social Care.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.E.H. carried out analyses, wrote the manuscript, reviewed and edited the manuscript, and contributed to the discussion. M.N. carried out analyses, reviewed and edited the manuscript, and contributed to the discussion. R.N.B. researched data, reviewed and edited the manuscript, and contributed to the discussion. O.T. carried out analyses and reviewed and edited the manuscript. B.M.S. was involved in the original data acquisition and analysis for EFSOCH, reviewed and edited the manuscript, and contributed to the discussion. D.M.S. was involved in the original HAPO analyses, reviewed and edited the manuscript, and contributed to the discussion. B.A.K. was involved in the original data acquisition and analysis for EFSOCH, reviewed and edited the manuscript, and contributed to the discussion. W.L.L. was involved in the original data acquisition and analysis for HAPO, reviewed and edited the manuscript, and contributed to the discussion. A.T.H. was involved in the original conception and design of EFSOCH, researched data, reviewed and edited the manuscript, and contributed to the discussion. R.M.F. researched data, wrote the manuscript, reviewed and edited the manuscript, and contributed to the discussion. A.T.H. (EFSOCH) and W.L.L. (HAPO) are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented at the Blair Bell Research Society Annual General Meeting at the Royal College of Obstetricians and Gynaecologists, London, U.K., 3 March 2017; the Diabetes UK Professional Conference, Manchester, U.K., 8–10 March 2017; and the British Maternal and Fetal Medicine Society 19th Annual Meeting, Amsterdam, the Netherlands, 30–31 March 2017.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db17-1188/-/DC1.
References
- 1.HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002 [DOI] [PubMed] [Google Scholar]
- 2.Straus DS. Growth-stimulatory actions of insulin in vitro and in vivo. Endocr Rev 1984;5:356–369 [DOI] [PubMed] [Google Scholar]
- 3.Pedersen J. Diabetes and Pregnancy: Blood Sugar of Newborn Infants Copenhagen, Danish Science Press, 1952 [Google Scholar]
- 4.Breschi MC, Seghieri G, Bartolomei G, Gironi A, Baldi S, Ferrannini E. Relation of birthweight to maternal plasma glucose and insulin concentrations during normal pregnancy. Diabetologia 1993;36:1315–1321 [DOI] [PubMed] [Google Scholar]
- 5.Sacks DA, Liu AI, Wolde-Tsadik G, Amini SB, Huston-Presley L, Catalano PM. What proportion of birth weight is attributable to maternal glucose among infants of diabetic women? Am J Obstet Gynecol 2006;194:501–507 [DOI] [PubMed] [Google Scholar]
- 6.Surkan PJ, Hsieh CC, Johansson AL, Dickman PW, Cnattingius S. Reasons for increasing trends in large for gestational age births. Obstet Gynecol 2004;104:720–726 [DOI] [PubMed] [Google Scholar]
- 7.Tyrrell J, Richmond RC, Palmer TM, et al.; Early Growth Genetics (EGG) Consortium . Genetic evidence for causal relationships between maternal obesity-related traits and birth weight. JAMA 2016;315:1129–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horikoshi M, Beaumont RN, Day FR, et al.; CHARGE Consortium Hematology Working Group; Early Growth Genetics (EGG) Consortium . Genome-wide associations for birth weight and correlations with adult disease. Nature 2016;538:248–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knight B, Shields BM, Hattersley AT. The Exeter Family Study of Childhood Health (EFSOCH): study protocol and methodology. Paediatr Perinat Epidemiol 2006;20:172–179 [DOI] [PubMed] [Google Scholar]
- 10.Abraham G, Inouye M. Fast principal component analysis of large-scale genome-wide data. PLoS One 2014;9:e93766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manichaikul A, Mychaleckyj JC, Rich SS, Daly K, Sale M, Chen WM. Robust relationship inference in genome-wide association studies. Bioinformatics 2010;26:2867–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laurie CC, Doheny KF, Mirel DB, et al.; GENEVA Investigators . Quality control and quality assurance in genotypic data for genome-wide association studies. Genet Epidemiol 2010;34:591–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urbanek M, Hayes MG, Armstrong LL, et al.; HAPO Study Cooperative Research Group . The chromosome 3q25 genomic region is associated with measures of adiposity in newborns in a multi-ethnic genome-wide association study. Hum Mol Genet 2013; 22:3583–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes MG, Urbanek M, Hivert MF, et al.; HAPO Study Cooperative Research Group . Identification of HKDC1 and BACE2 as genes influencing glycemic traits during pregnancy through genome-wide association studies. Diabetes 2013;62:3282–3291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol 2003;189:1698–1704 [DOI] [PubMed] [Google Scholar]
- 16.Silverman BL, Rizzo TA, Cho NH, Metzger BE. Long-term effects of the intrauterine environment. The Northwestern University Diabetes in Pregnancy Center. Diabetes Care 1998;21(Suppl. 2):B142–B149 [PubMed]
- 17.Bjӧrntorp P. Metabolic implications of body fat distribution. Diabetes Care 1991;14:1132–1143 [DOI] [PubMed] [Google Scholar]
- 18.McCowan LME, Thompson JMD, Taylor RS, et al.; SCOPE Consortium . Prediction of small for gestational age infants in healthy nulliparous women using clinical and ultrasound risk factors combined with early pregnancy biomarkers. PLoS One 2017;12:e0169311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viera MC, McCowan LME, Gillett A, et al.; SCOPE Consortium . Clinical, ultrasound and molecular biomarkers for early prediction of large for gestational age infants in nulliparous women: an international prospective cohort study. PLoS One 2017;12:e0178484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitzman JO, Snyder MW, Ventura M, et al. Noninvasive whole genome sequencing of a human fetus. Sci Tranl Med 2012;4:137–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiorentino F, Bono S, Pizzuti F, et al. The clinical utility of genome-wide non invasive prenatal screening. Prenat Diagn 2017;37:593–601 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.