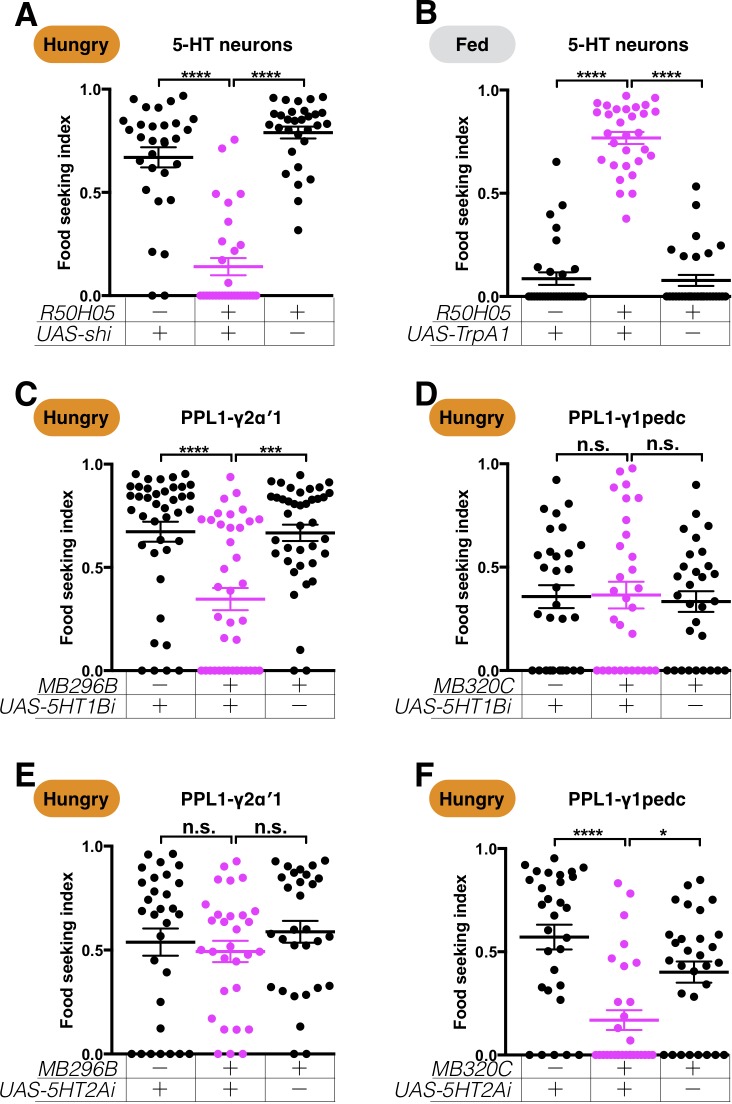
Figure 10. Serotonin regulates PPL1-γ2α′1 and PPL1-γ1pedc DANs via different receptors.
The yeast food-seeking performance of 24-hr-starved (A and C–F) and food-satiated (B) male flies was assessed. (A) The performance of R50H05-GAL4;UAS-shits1 flies was statistically worse than for the control flies at a restrictive 32°C (5-HT neurons, Kruskal-Wallis, n = 30, p<0.0001). (B) The performance of R50H05-GAL4;UAS-TrpA1 flies was statistically better than for the control flies at a restrictive 32°C (5-HT neurons, Kruskal-Wallis, n = 30, p<0.0001). (C) The performance of MB296B;UAS-5HT1B-RNAi flies was statistically worse than for the control flies (PPL1-γ2α′1, Kruskal-Wallis, n = 39–40, p=0.0003). (D) The performance of MB320C;UAS-5HT1B-RNAi flies was not statistically different from that of control flies (PPL1-γ1pedc, Kruskal-Wallis, n = 30, p>0.9999). (E) The performance of MB296B;UAS-5HT2A-RNAi flies was not statistically different from that of control flies (PPL1-γ2α′1 Kruskal-Wallis, n = 30, p=0.5116). (F) The performance of MB320C;UAS-5HT2A-RNAi flies was statistically worse than that of control flies (PPL1-γ1pedc, Kruskal-Wallis, n = 30, p=0.0263). Individual data points and mean ± SEM are shown.