Figure 1. Conservation of canonical circadian clock from invertebrates (A) to vertebrates (B).
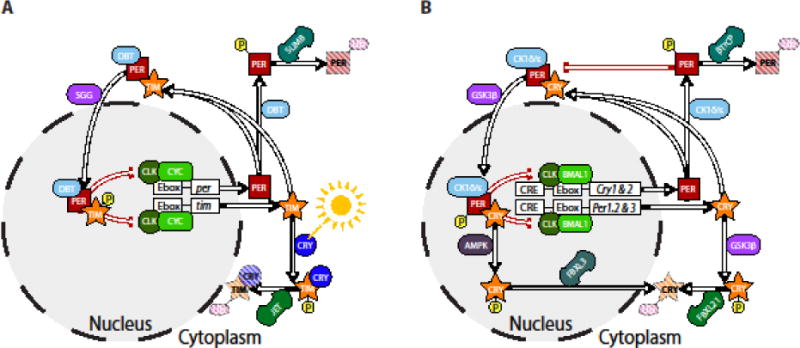
A) In a highly simplified model of the invertebrate circadian clock Clock and cycle genes drive transcription of period and timeless via enhancer boxes (Ebox) found upstream of the genes. PER and TIM proteins form heterodimers which translocate back to the nucleus to inhibit their own translation. While CRY-mediated ubiquitination and subsequent proteosomal degradation of TIM drives daily resetting by light, clock speed is controlled by the rate of degradation of PER proteins. B) Similar feedback and cycling occurs in mammals with the Cryptochrome genes substituting for timeless in the core oscillator. Cytosolic PER and CRY proteins are ubiquitinated by two separate mechanisms when not heterodimerized. PER is flagged for degradation by the doubletime orthologues CK1δ/ε leading to ubiquitination by βTrCP, while phase-resetting occurs via intracellular signaling pathways AMPK (Cry1) and GSK3β (Cry2) triggering FBXL3 ubiquitination and proteosomal degradation mediated intracellular signaling. In these figures, several accessory loops in both flies and mammals are omitted to focus on the balance of synthesis and degradation of clock proteins, which has been implicated in the circadian timing of sleep in humans. Indeed, all mammalian genes presented here, except Clk and Bmal1, have at least one allelic variant associated with a sleep-wake disorder. Abbreviations: AMPK - AMP-activated protein kinase; BMAL1 – Brain and muscle arntl-like protein 1; βTrCP – β-transducin repeat-containing protein; CK1δ/ε – Casein kinase 1 delta/epsilon; CLK – Clock; CRE – cAMP response element; CRY – Cryptochrome; CYC – Cycle; Ebox – promoter enhancer box; FBXL – F-box and leucine rich repeat protein ; GSK3β – Glycogen synthase kinase-3; PER – Period; SGG – Shaggy; TIM – Timeless; Ub – post-translational ubiquitination.
