Fig. 7.
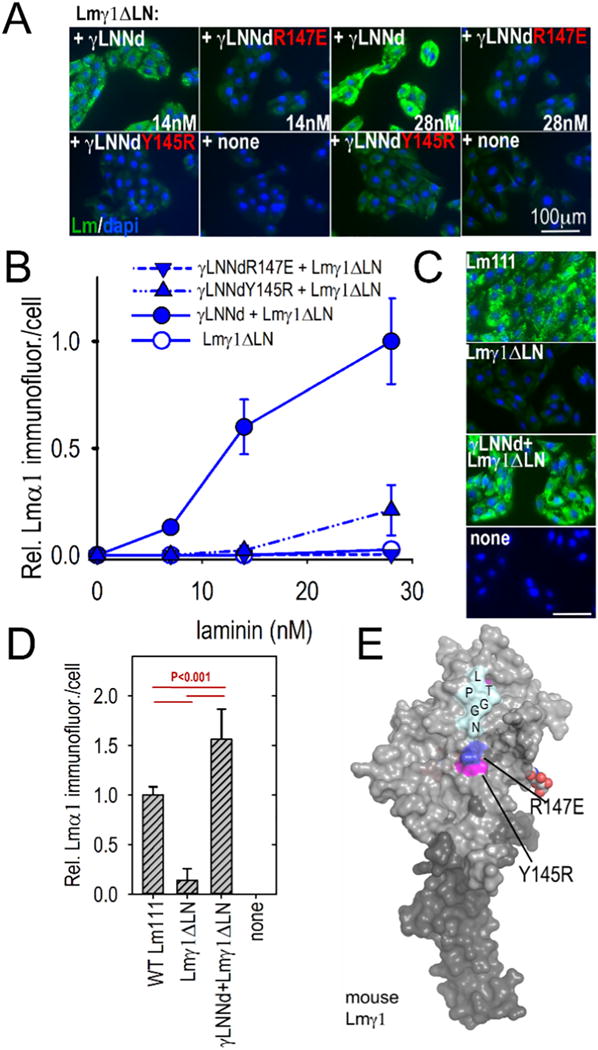
Assembly of γLNNd–Lmγ1ΔLN complexes on cell surfaces. Panels A–B. γLNNd proteins, WT and bearing LN point mutations and coupled to Lmγ1ΔLN, were added to the medium of SCs for 1 h, washed, fixed and immunostained to detect cell-adherent laminin through detection of α1 LG domains. For the concentration series, representative images and plots of average (±S.D., n = 6–7 fields) intensity/cell as a function of laminin/γLNNd concentration are shown. Laminin assembly on SCs was abolished by R147E and almost completely abolished by Y145R. Panels C–D. WT Lm111 accumulation was compared to that of the γLNNd-Lmγ1ΔLN complex at 28 nM laminin. Panel E. Surface contour of mouse laminin γ1LN and adjacent LEa domain segment with attached N-linked carbohydrates showing location of the mutations examined in γLNNd proteins (Pymol surface contour rendition of mouse Lmγ1, PDB # 4AQT, [36]).
