Abstract
Background and Purpose
Nearly a quarter of those in the US over age 71 experience mild cognitive impairment (MCI). Persons with MCI (PwMCI) battle depression and progressive disengagement from daily activities, which contribute to participation restriction and activity limitation. Daily engagement in meaningful activity (DEMA) is a tailored intervention designed to benefit PwMCI and their caregivers through preserved engagement and supported adjustment to cognitive changes. This secondary analysis was guided by the International Classification of Functioning, Disability and Health (ICF) model. Aims were to (i) explore the extent to which change in self-rated activity performance and physical function can predict change in depressive symptoms, (ii) evaluate for difference in confidence and depressive symptoms at ICF levels of activity and participation, and (iii) quantify the impact of daily engagement at the ICF level of participation on physical function.
Methods
A secondary analysis was conducted using data from the parent study, which was a two-group randomized trial involving PwMCI and their informal caregivers participating in the Indiana Alzheimer Disease Center DEMA program. Quantitative analysis (dyads: DEMA N = 20, Information Support N = 20) examined outcomes at posttest and follow-up. Analysis employed linear regression to model the relationship between explanatory and dependent variables and independent t-test to examine for difference in confidence, depression, and physical function.
Results and Discussion
At posttest, change in self-rated performance predicted change in depressive symptoms. Those in the DEMA group who engaged in activity at the ICF level of participation demonstrated a significant increase in confidence and physical function. Although not significant, the control group posttest results showed a mean decrease in confidence.
Conclusions
Results demonstrate a positive impact of DEMA on depressive symptoms, confidence, and physical function. Change in occupational performance predicted change in depressive symptoms. Confidence significantly improved among those who engaged at the ICF participation level. A larger, randomized controlled longitudinal trial is needed to better assess the impact of DEMA on physical function, activity, participation restriction and quality of life.
Keywords: older adults, mild cognitive impairment, physical function, depression, confidence
INTRODUCTION
Mild cognitive impairment (MCI) is understood as a transitional state between age-related cognitive changes and the earliest behavioral and clinical signs of Alzheimer’s Disease (AD).1 In the United States, nearly a quarter of those over age 71 experience MCI.2 Cognitive decline is an independent risk factor for falls and persons with MCI (PwMCI) report more than 3 times more hospitalizations than those in the same age group who are hospitalized for causes other than MCI.3,4 Hallmarks of MCI include, but are not limited to, elevated risk of emotional distress, depressive symptoms and decline in physical function.5 Studies of community-dwelling PwMCI reveal a nearly 20% prevalence of depression, markedly higher than the 1 – 5% average among community-dwelling older adults without a diagnosis of MCI.6,7 Moreover, cognitive decline and diminished confidence adversely impact self-care behaviors, whereas depressive symptoms predict decreases in instrumental activities of daily living (IADL), such as the ability to handle finances, the ability to shop, and independently use transportation.8,9 Absence of a cure for MCI highlights the urgency to implement innovative, multifaceted interventions that are reproducible and scalable.
Little is known about high-impact, non-pharmacologic interventions that optimize remaining abilities and, potentially, attenuate functional decline. The literature supports the value of structured interventions such as the daily engagement meaningful activity (DEMA) program, which is a strengths-based, multicomponent, family-centered, tailored intervention designed to benefit persons with MCI and their caregivers. 10,11 The DEMA study was a NIH-funded (R21NR013755), 2-group randomized, pre-post intervention controlled pilot study with 3 data collections over 6 months. The aim of the study was to evaluate feasibility, satisfaction, and preliminary effects of the DEMA intervention in 40 MCI dyads.10–13 Briefly, 40 MCI patient-caregiver dyads were stratified by Patient Health Questionnaire-9 (PHQ-9) scores (≤4 or ≥ 5, respectively)14 and stage of MCI (early or late), then randomized to either the DEMA program or an Information Support (IS) attention control group. After randomization, both study arms received 6 bi-weekly sessions (2 sessions face-to-face and 4 via phone) over 3 months, followed by 2 additional measurement sessions.
Preliminary findings from the parent study demonstrated high feasibility, acceptability and provided preliminary effect size data. The parent DEMA study results indicated that DEMA may have greater effect in the health related outcomes of PwMCI with depressive symptoms (PHQ-9 ≥5) such as physical function and mood.11–13 From a physical therapy perspective, understanding that activity engagement can positively impact MCI-related symptoms is informative. However, the predictors of change in confidence and depression, which would significantly benefit physical therapy intervention design, are still not understood. Moreover, there is opportunity to employ the International Classification of Functioning, Disability and Health (ICF) model15 to better discern the impact of engagement in meaningful activities on physical function at the participation level. Addressing this information gap will inform and support physical therapists’ intervention approaches to improving PwMCI function and quality of life. Therefore, the present study was designed as a secondary analysis of the DEMA parent study with aims to (i) explore the extent to which change in self-rated activity performance and physical function can predict change in depressive symptoms, (ii) evaluate for difference in confidence and depressive symptoms at ICF levels of activity and participation, and (iii) quantify the impact of daily engagement at the ICF level of participation on physical function.
METHODS
To be eligible for the parent DEMA study, both PwMCI and caregivers were required to consent to participate and possess a working phone in the home or daily access to a telephone. PwMCI inclusion criteria were: 1) aged 60 years or older; 2) spoke English; 3) presented with both caregiver-reported, clinically significant decline in cognition and practitioner-detected cognitive impairment on the standardized health exam; 4) presented with at least one cognitive assessment score below the 7th percentile; and 5) presented in the normal range in performance of daily living tasks based on informant interview information, which indicated that impairment did not rise to the level of dementia. Family caregivers were eligible providing they: 1) were adults ≥ 21 years of age; 2) had primary responsibility for providing unpaid care to a PwMCI, along with monitoring for safety and providing social support; 3) were able to read and speak English; and 4) were oriented to person, place, and time (that is, had a Six-Item Screener to Identify Cognitive Impairment, score >4).16 PwMCI and family caregivers were excluded if: 1) the PwMCI or caregiver presented with a diagnosis of bipolar disorder or untreated schizophrenia; or 2) the caregiver had significant cognitive impairment that would hinder participation (a Six-Item Screener < 5).16
In the parent study, MCI participant and caregiver dyads were randomized to 1 of 2 standardized conditions: DEMA or Information Support (IS) attention control with 4 strata based on PwMCIs’ depression scores (PHQ-9 ≤4 vs. PHQ-9 ≥5) and cognitive status using the Mini-Mental Status Examination (MMSE≤ 23 vs. MMSE ≥ 24 ).17 Immediately after consent, eligible dyads completed the pre-intervention data collection (baseline) and were then randomized. Following randomization, both groups received 7, 60-minute sessions (2 face-to-face, 4 via telephone) over 12 weeks.
The DEMA sessions were delivered by a trained registered nurse intervener who worked with the MCI dyads to develop and implement goals related to self-identified meaningful activity. Each session had 2 components. First, MCI dyads were guided to use the steps of problem-solving therapy to review and plan self-selected meaningful activities. Second, the intervener and dyad discussed 1 of the 6 topics in Self-Management Tool kit relevant to understanding MCI, its treatments, management and other resources. The Self-Management Tool kit supports independent use of self-management skill-building between MCI dyads.
Dyads randomized to the IS attention control received the American Alzheimer Association MCI pamphlet. During sessions, nurse interveners provided support using active listening strategies and opportunities for participants to 1) ask questions related to the MCI pamphlet or other health questions; 2) express their activity engagement experiences, and 3) encourage the dyads to seek additional information from the Alzheimer Association or their healthcare providers as needed.
Data collections were conducted by trained evaluators via private telephone interview at pre-intervention (baseline), immediately after (posttest) and again at 3 months (follow-up) post-intervention. All participants, PwMCI and informal caregivers, were interviewed separately.
Approval for this secondary analysis was granted by the Indiana University Purdue University at Indianapolis Institutional Review Board. Secondary analysis was conducted on PwMCI DEMA and IS attention control group data that were extrapolated from the parent study baseline, posttest and follow-up measures. For aim 2, data were further sub-grouped by ICF15 activity-level (in-home, individual execution of a task or action) or participation-level (community, involvement in life situations) based on primary target activity selection. For example, engaging in activities such as playing the piano or re-organizing a room were categorized at the ICF activity level whereas attending a grandchild’s ballgame or going out to eat with friends were categorized at the participation level.
Measures
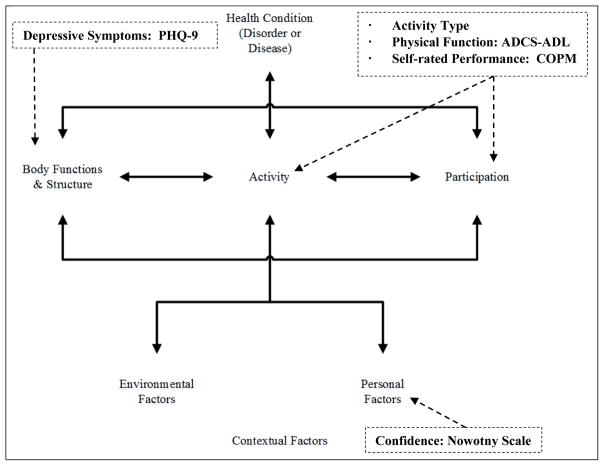
Figure 1 depicts affiliation of secondary analysis measures to partial components of the ICF model. Depressive symptoms were measured by the Patient Health Questionnaire (PHQ-9),14 while confidence, self-rated activity performance, and physical function were measured respectively by the Nowotny Confidence Scale,18 the Canadian Occupational Performance Measure (COPM)19, and the Alzheimer Disease Cooperative Study – Activities of Daily Living Inventory (ADCS-ADL).20 As verified in the parent study,11 the outcome instruments included in this secondary analysis demonstrate sufficient psychometric properties, including acceptable internal consistency coefficient (ICC), sensitivity to change, and reasonable response burden.
Figure 1. Secondary analysis measures as guided by the ICF model.
Call-out box contents depict secondary analysis study variable affiliations to the ICF model. ICF: International Classification of Functioning, Disability and Health; PHQ-9: Patient Health Questionnaire-9; Activity Type: study participant self-selected primary target activity selection categorized by either ICF activity or participation level; ADCS-ADL: Alzheimer-Disease Cooperative Study-Activites of Daily Living Inventory; COPM: Canadian Occupational Performance Measure.
The PHQ-9 is the depression module of the Patient Health Questionnaire (PHQ) with ICC = 0.83 to 0.92.14 Scores indicate severity of depressive symptoms as minimal (1–4), mild (5–9), moderate (10–14), moderately severe (15–19), and severe (20–27).14 The Notwotny Confidence Scale is a subscale of the Nowotny Hope Scale that uses a 4-point Likert response format of strongly agree to strongly disagree to self-report confidence in one’s own ability (ICC = 0.83 to 0.92).18 The COPM measures a person’s self-perceived experiences of occupational performance (interface between the person and environment) through measurement of a client’s ratings of activity, satisfaction, and performance as correlated to self-care, productivity, and leisure (ICC = 0.86 to 0.95).19 Clients identify their most important problems in occupational performance and provide a 0 to 10 score for both performance and satisfaction. Higher scores indicate greater activity performance and satisfaction. The ADCS-ADL is a 24-item instrument that assesses physical functional ability with everyday tasks on the basis of informant ratings of client performance. Informants are directed to focus on the past 4 weeks and on what the patient actually did as opposed to estimating what the patient might be able to do (ICC = 0.91).20 In-depth review of the instrument revealed a 3-item subset that corresponded to the ICF participation level. Examination of a composite sub-score would reveal the impact of engagement on physical function at the ICF participation level.
Statistical Analysis
Secondary analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, North Carolina). PwMCI demographic characteristics were summarized using descriptive statistics. To satisfy aim 1, simple linear regression was performed in order to understand the extent to which change in self-rated activity performance and physical function predict change in depressive symptoms. Of the 20 dyads originally randomized to the DEMA group, 1 passed away, 1 progressed to AD and 1 withdrew due to caregiver illness prior to posttest and follow-up data collection. The number of the DEMA group post-intervention observations were then limited to 16, rendering multiple regression unrealistic because of limited statistical power. Based on change in each predictor variable (confidence, COPM, or ADCS-ADL), separate linear regressions were calculated to predict change in depressive symptoms. The t-values were test statistics required for each parameter whereby p-values were calculated. For aim 2, DEMA and IS participants were sub-grouped into an ICF15 activity level (in-home, individual execution of a task or action) or participation level (community, involvement in life situations) category based on primary target activity selection. Data were examined for posttest and follow-up difference in confidence and depressive symptoms using the dependent t-test. This was conducted separately for the DEMA and IS group. The number of observations that were used for each linear regression depended on non-missing values of dependent variables and independent variables. Therefore, the degrees of freedom (DF) were different for each linear regression. To satisfy aim 3, the independent sample t-test was performed in order to quantify the impact of engagement at the ICF participation level on physical function. A composite sub-score for the ADCS-ADL was calculated using a 3-item subset (8, 11, 15) that corresponded to function at the ICF participation level. Questions 8 (In the past 4 weeks, did [Subject’s Name] keep appointments or meetings with other people, such as relatives, a doctor, the hairdresser, etc.?), 11 (In the past 4 weeks, did [Subject’s Name] get around [or travel] outside of his/her home?), and 15 (In the past 4 weeks, did [Subject’s Name] ever go shopping at a store?) comprised the sub-score calculation. “Yes” responses to questions 8 and 11 availed 3 points each and question 15 availed 1 possible point. Calculation yielded a composite sub-score of 7 possible points. The ADCS-ADL sub-score was then assessed for difference posttest from baseline. For all analyses, findings demonstrating a p value less than .05 were considered statistically significant.
RESULTS
Demographic characteristics of PwMCI DEMA and IS attention control group subjects are summarized in Table 1. The majority were male, mostly Caucasian and most PwMCI initially presented in late stage MCI with a PHQ-9 score less than 5.11
Table 1.
Demographic Characteristics of Study PwMCI at Baseline
| Group | DEMA N=20 |
IS N=20 |
p value |
|---|---|---|---|
| Age, y | 71.2 | 76.5 | |
| Mean (SD) | (6.8) | (7.1) | 0.02* |
|
| |||
| Gender, N (%) | |||
| Male | 12 (60.0) | 11 (55.0) | 0.75 |
| Female | 8 (40.0) | 9 (45.0) | |
|
| |||
| Race, N (%) | |||
| Caucasian | 16 (80.0) | 19 (95.0) | |
| African American | 4 (20.0) | 1 (5.0) | |
| Asian | 0 | 0 | |
| More than 1 Race | 0 | 0 | 0.34 |
|
| |||
| Education, y | 16.8 | 16.15 | |
| Mean (SD) | (4.1) | (3.9) | 0.61 |
|
| |||
| Employment, N | |||
| Employed Full Time | 2 (10.0) | 1 (5.0) | |
| Employed Part Time | 1 (5.0) | 1 (5.0) | |
| Retired | 17 (85.0) | 18 (90.0) | |
| Not Employed | 0 | 0 | >0.99 |
|
| |||
| MCI Stage, N (%) | |||
| Early MCI | 8 (40.0) | 10 (50.0) | |
| Late MCI | 12 (60.0) | 10 (50.0) | 0.53 |
|
| |||
| PHQ-9 (≥ 5), N (%) | |||
| Yes | 7 (35.0) | 8 (42.1) | |
| No | 13 (35.0) | 11 (57.9) | 0.65 |
p ≤ 0.05.
PwMCI: persons with mild cognitive impairment; DEMA: daily engagement in meaningful activity; IS: information support attention control group.
Depressive Symptoms and Confidence
The posttest and follow-up linear regression findings specific to aim 1 (explore the extent to which change in self-rated activity performance and physical function predict change in depressive symptoms) are shown in Table 2. From baseline to posttest, change in self-rated activity performance significantly (COPM: p = 0.03) predicted change in self-reported depressive symptoms (PHQ-9). As self-rated occupational performance increased, depressive symptoms decreased. Follow-up results, however, were not statistically significant.
Table 2.
Posttest and Follow-up from Baseline DEMA Change in Confidence, COPM, ADCS-ADL Prediction of Change in Depressive Symptoms
| Posttest(N = 17) | Follow-up(N = 16) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Effect | Estimate | Standard Error | DFa | t value | p value | Estimate | Standard Error | DFa | t value | p value |
| Intercept | −0.2 | 0.9 | 15 | −0.2 | 0.82 | −1.5 | 0.7 | 14 | −2.1 | 0.05 |
| Change of Confidence | 0.1 | 0.2 | 15 | 0.4 | 0.66 | −0.6 | 0.3 | 14 | −2.1 | 0.06 |
| Intercept | 0.2 | 0.7 | 10 | 0.2 | 0.82 | −1.5 | 0.8 | 13 | −1.9 | 0.08 |
| Change of COPM | −1.4 | 0.6 | 10 | −2.5 | 0.03* | −0.1 | 0.4 | 13 | −0.2 | 0.84 |
| Intercept | −0.1 | 0.9 | 14 | −0.1 | 0.94 | −2.3 | 0.8 | 13 | −2.7 | 0.02* |
| Change of ADCS-ADL | 0.1 | 0.2 | 14 | 0.3 | 0.74 | −0.2 | 0.2 | 13 | −1.3 | 0.23 |
p ≤ 0.05.
Posttest = 2 weeks post intervention; Follow-up = 3 months post.
DEMA: daily engagement in meaningful activity; COPM: Canadian Occupational Performance Measure; ADCS-ADL: Alzheimer Disease Cooperative Study – Activities of Daily Living Inventory.
The number of observations that were used depended on non-missing values of dependent and independent variables. Therefore, the DF were different.
In the DEMA group, change in self-rated activity performance (COPM), physical function (ADCS-ADL), and confidence (Nowotny Confidence Scale), did not predict change in self-reported depressive symptoms (PHQ-9). Change in confidence fell just short of statistical significance (p = 0.06).
Results for aim 2 (evaluate for difference in confidence and depressive symptoms at ICF levels of activity and participation) are shown in Table 3. Those in the DEMA group who initially engaged in activities at the ICF participation level demonstrated a significant difference in confidence (p = 0.01). Although not significant, the IS group posttest results showed a mean decrease in confidence. At follow-up, the DEMA model could not be analyzed because of the limited number of participants, and change in IS data was not significant (p = 0.31).
Table 3.
Difference in Confidence - Posttest and Follow-Up from Baseline ICF Participation Levela in DEMA and IS Group
| Posttest | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group | N | Baseline Mean (SD) | Posttest/Follow-up Mean (SD) | Mean Difference | DFb | Standard Error | t value | p value |
| DEMA | 7 | 26.6 (1.2) | 28.4 (0.8) | −1.8 | 12 | 0.6 | −3.2 | 0.01* |
| IS | 8 | 25.5 (1.2) | 25.0 (1.2) | 0.6 | 15 | 1.0 | −0.3 | 0.77 |
| Follow-up | ||||||||
| DEMA | 4 | NA | NA | NA | NA | NA | NA | NA |
| IS | 13 | 25.0 (1.1) | 26.0 (1.4) | −1.1 | 16 | 1.0 | −1.1 | 0.31 |
p ≤ 0.05
ICF: International Classification of Functioning; DEMA: daily engagement in meaningful activity; IS: Information Support attention control group.
DEMA and IS participants were sub-grouped into an ICF activity/participation level category based on their primary target activity selection.
DF based on dependent t-test, conducted separately for the DEMA and IS group. Posttest DEMA DF adjusted due to a missing value.
As seen in Table 4, the DEMA PHQ-9 mean score decreased by 1.86 points from baseline to posttest, whereas the IS mean score increased by 0.26. Although neither change was significant, the DEMA group mean posttest score reflected a categorical shift from “mildly depressed” (≥ 5 = mildly depressed) to “minimally depressed” (1–4 = “minimally depressed” and 0 = “none”).14 Although follow-up findings were not statistically significant, the DEMA group remained in the “minimally depressed” category.
Table 4.
Difference in Depressive Symptoms-Posttest and Follow-up from Baseline ICF Participation Level in DEMA and IS Group
| Posttest | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group | N | Baseline Mean (SD) | Posttest/Follow-up Mean (SD) | Mean Difference | DFa | Standard Error | t value | p value |
| DEMA | 17 | 6.3 (1.3) | 4.5 (1.9) | 1.9 | 12 | 2.5 | 0.7 | 0.48 |
| IS | 16 | 3.6 (0.8) | 3.9 (1.3) | −0.3 | 15 | 1.0 | −0.3 | 0.80 |
| Follow-up | ||||||||
| DEMA | 17 | 6.1 (1.3) | 1.8 (1.8) | 4.3 | 11 | 2.2 | 2.0 | 0.07 |
| IS | 18 | 3.4 (0.8) | 2.7 (0.7) | 0.8 | 16 | 0.8 | 0.9 | 0.36 |
ICF: International Classification of Functioning; DEMA: daily engagement in meaningful activity; IS: Information Support control group. Posttest = 2 weeks post intervention; Follow-up = 3 months post intervention.
The number of observations that were used depended on non-missing values of dependent and independent variables. Therefore, the DF were different.
Examination for difference in confidence and depressive symptoms was repeated at the ICF activity level for both groups. Posttest IS models for confidence and depressive symptoms could not be run because of the limited sample size and a convergence issue. Neither the IS nor DEMA group data yielded significant findings for ICF activity level at follow-up.
Change in Physical Function
Specific to aim 3 (quantify the impact of daily engagement at the ICF level of participation on physical function), the DEMA group (Table 5) demonstrated significant improvement in the ICF participation level sub-score of the ADCS-ADL (posttest mean difference = 0.15, p = 0.04). Data did not demonstrate continuance of significant difference through follow-up.
Table 5.
ADCS-ADL Sub-Composite Score Change Posttest and Follow-up from Baseline in DEMA and IS Group
| ADCS-ADL sub-score change posttest from baseline | ||||||
|---|---|---|---|---|---|---|
| Time Point | N | Mean (SD) | Mean Difference | Standard Error | t value | p value |
| IS Change | 18 | 2.9 (0.3) | −0.1 | 0.1 | −0.9 | 0.39 |
| DEMA Change | 16 | 3.0 (0.0) | 0.2 | 0.01 | 2.1 | 0.04* |
| DEMA vs IS Change | 0.2 | 0.1 | 2.1 | 0.04* | ||
| ADCS-ADL sub-score change follow-up from baseline | ||||||
| IS Change | 18 | 2.9 (0.2) | −0.1 | 0.1 | −0.6 | 0.56 |
| DEMA Change | 16 | 2.9 (0.3) | 0.04 | 0.1 | 0.4 | 0.70 |
| DEMA vs. IS | 0.1 | 0.1 | 0.7 | 0.49 | ||
p-value significant ≤ 0.05
ADCS-ADL: Alzheimer Disease Cooperative Study – Activities of Daily Living Inventory; DEMA: daily engagement in meaningful activity; IS: Information Support control group. Posttest = 2 weeks post intervention; Follow-up = 3 months post intervention.
DEMA participants at the ICF participation level significantly improved from baseline to posttest in physical function (ADCS-ADL), whereas the IS group demonstrated a sub-score decrease. The data also demonstrated a significant difference in participation sub-score change between the DEMA and IS groups, although this difference did not continue through follow-up.
DISCUSSION
Focused on PwMCIs’ response to a non-pharmacologic, innovative intervention of daily engagement in meaningful activity, this secondary analysis is the first to explore the following aims in light of partial components of the ICF model: (i) examine the predictors of change in self-reported depressive symptoms, (ii) evaluate for difference in confidence and depressive symptoms by ICF subgrouping, and (iii) quantify the impact of engagement on physical function at ICF level of participation. Similar to other activity-focused and behavioral intervention studies for persons with dementia and PwMCI, this study sample was comprised of adults over 60 years of age who presented with a confirmed diagnosis of MCI and benefited from the assistance of a vested, informal caregiver.21 Although there was a significant difference in age between the DEMA and IS groups, both IS and DEMA means were in the 70–79 cohort parameters commonly employed by the World Health Organization and National Institute on Aging.22
Depressive Symptoms and Confidence
From baseline to posttest, DEMA change in self-rated activity performance (COPM) significantly predicted change in depressive symptoms (PHQ-9). As self-rated occupational performance improved depressive symptoms decreased. PwMCI in the DEMA group self-selected primary target activities that reflected personal, priority values and tailored goals, thereby optimizing perceived control. Perceived control and self-rated improvement is linked to increasing confidence, which the literature identifies as a mediator for outcomes such as change in depressive symptoms.23 Moreover, O’Shea and colleagues propose that self-efficacy (confidence) moderates the relationship between self-rated memory function and depressive symptoms.24 Despite a notable shift in the DEMA group mean score from baseline to posttest, change in confidence did not predict change in depressive symptoms. This may have been because of the limited sample size. Because the feasibility study sample was further sub-grouped by intervention and control, intermittent challenges to statistical power were not surprising.
Although current findings demonstrate change in depressive symptoms, it is not clear to what extent or how confidence influenced this change. The potential moderating role of confidence needs to be evaluated further via a longitudinal study with a larger sample size.
At posttest, DEMA participants allocated to the ICF participation subgroup demonstrated a significant increase in confidence. Recent studies confirm the value of engagement at the ICF participation level for preserving cognitive abilities and independence with IADL.25,26 Moreover, studies designed to address chronic health conditions with co-morbid depression and/or cognitive impairment indicate that self-efficacy, or task-specific confidence, plays a significant role in behaviors such as self-care and on-going self-management.27,28 Although not statistically significant, the DEMA subgroup at the ICF participation level did shift at posttest and follow-up from the “mildly depressed” mean at baseline to “minimally depressed.” The absence of statistical significance could be because of the small sample size; however, the categorical shift in depressive symptoms (PHQ-9) is considered clinically relevant and meaningful.14,29 The disability literature, along with aging studies, affirm that participation is a primary goal for persons with disability and an important health outcome across the prevention spectrum.29 Likewise, by-products of activity at the ICF participation level, such as enhanced social support, reduce the risk for depression in older adults.30
Change in Physical Function
The improvement in physical function (ADCS-ADL) at the ICF participation level (keeping appointments, getting around outside home, going shopping at a store) was significant for DEMA participants. These findings for PwMCI are consistent with a study that found a positive impact of participation outside the home on IADL in community-dwelling older adults.26 Diminishing engagement in activities that involve “high cognitive demand,” such as shopping, is strongly associated with MCI,31 further emphasizing both the functional and quality of life significance of the participation sub-score findings.
Physical Therapy Implications
Nearly 1 in 4 persons over the age of 65 presents with MCI, impacting daily function and capability to participate in life and societal roles.31,32 Research indicates the value of exercise in persons battling cognitive changes.33 Yet, front-line clinicians must navigate significant barriers to exercise adherence such as declining short-term memory and confidence, as well as changes in motivation and emotional affect. Such factors negatively impact capacity to recall routines and perform home exercises as prescribed, thereby hindering skill acquisition and functional improvement.33
The findings from this secondary analysis provide valuable insights relative to physical therapy. Change in self-rated activity performance predicted change in self-reported depressive symptoms. Though performance measures are key to quantifying functional progress, this finding indicates the importance of incorporating patient perceptions. Moreover, PwMCI in the DEMA group self-selected their primary target activity. Although self-selection of all exercises may not be optimal, including the patient in the process of exercise prescription promotes a sense of control and ownership while encouraging dialogue that helps both practitioner and patient anticipate and navigate challenges to adherence.
Confidence significantly improved among those who engaged at the ICF participation level. In contrast, physical therapy home programs commonly address underlying impairments, such as strength, balance, and physiologic reserve, independently. Incorporation of participation level elements into the home program fosters confidence. For example, prescribed activities such as walking should be adjusted by working with the patient and informal caregiver to schedule, map out, and walk short distances at the mall or grocery store. Moreover, the physical therapist should work with the patient to identify and plan self-rewards for achieving exercise adherence and goals, such as an outing with friends. Simple adjustments such as these help re-connect the patient to life roles and social support while expanding functional capability and confidence.
Current US public health policies and plans advocate for innovative dementia-capable efforts across the preventive spectrum.34 The parent study was a NIH-funded two-group randomized trial intended to ascertain the feasibility, effect size, acceptability, and usefulness of the DEMA program for PwMCI. Parent study findings are promising10,11,35 and indicate areas for program refinement and expansion. Studies that directly address multicomponent interventions for PwMCI20 are rare, making the opportunity to conduct a secondary analysis and capture additional insight from outcome data not only prudent, but imperative.
There were several limitations of this study. First, the sample size was small. In the future, a larger, more adequately powered randomized controlled longitudinal clinical trial is needed to further evaluate impact of perpetual activity engagement. Second, due to potential biases introduced by self-selection, the study has limited generalizability to the broad-spectrum population of MCI patients in MCI-caregiver dyads. Future research is recommended that includes more ethnically diverse PwMCI and informal caregivers.
CONCLUSIONS
Results clearly indicate the positive impact of the DEMA program. Future research should explore the extent to which the DEMA program impacts physical function as well as activity and participation in the home and community. Second, future research should explore directly whether the DEMA intervention reduces participation restriction in PwMCI and leads to improved quality of life outcomes. Third, opportunity exists to enhance interdisciplinary collaboration aimed at catalyzing replicability, scalability and multi-setting implementation of this strengths-based, multicomponent, tailored intervention.
Acknowledgments
The parent study was supported by grant funding from NIH/NINR R21NR013755 (PI: Y. Lu).
The authors thank the Indiana Alzheimer’s Disease Center’s support of subject recruitment for the parent study (IADC funding source: P30AG010133-22, NIA), and Ziyi Yang for her consultation during secondary data analysis.
Footnotes
The authors declare no conflict of interest.
References
- 1.Peterson RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 2.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148(6):427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alzheimer Association. Characteristics, costs and health service use for Medicare beneficiaries with a dementia diagnosis: Report 1: Medicare current beneficiary survey. Chicago: Alzheimer’s Association; 2009. Alzheimer’s Association. [Google Scholar]
- 4.Amboni M, Barone P, Hausdorff JM. Cognitive contributions to gait and falls: Evidence and implications. Mov Disord. 2013;28(11):1520–1533. doi: 10.1002/mds.25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown PJ, Devanand DP, Liu X, Caccappolo E. Functional impairment in elderly patients with mild cognitive impairment and mild Alzheimer disease. Arch Gen Psychiatry. 2011;68(6):617–626. doi: 10.1001/archgenpsychiatry.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zahodne LB, Devanand DP, Stern Y. Coupled cognitive and functional change in Alzheimer’s disease and the influence of depressive symptoms. J Alzheimers Dis. 2013;34(4):851–860. doi: 10.3233/JAD-121921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC. [Accessed 06/17, 2016];Depression is not a normal part of growing older. 2016 At http://www.cdc.gov/aging/mentalhealth/depression.htm.
- 8.Perneczky R, Pohl C, Sorg C, et al. Impairment of activities of daily living requiring memory or complex reasoning as part of the MCI syndrome. Int J Geriatr Psychiatry. 2006;21(2):158–162. doi: 10.1002/gps.1444. [DOI] [PubMed] [Google Scholar]
- 9.Vellone E, Fida R, D’Agostino F, et al. Self-care confidence may be the key: A cross-sectional study on the association between cognition and self-care behaviors in adults with heart failure. Int J Nurs Stud. 2015;52(11):1705–1713. doi: 10.1016/j.ijnurstu.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Lu YY, Haase JE, Weaver M. Pilot testing a couples-focused intervention for mild cognitive impairment. J Gerontol Nurs. 2013;39(5):16–23. doi: 10.3928/00989134-20130403-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu YY, Bakas T, Yang Z, Weaver MT, Austrom MG, Haase JE. Feasibility and effect sizes of the revised daily engagement of meaningful activities intervention for individuals with mild cognitive impairment and their caregivers. J Gerontol Nurs. 2016;42(3):45–58. doi: 10.3928/00989134-20160212-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu YY, Haase JE. Content validity and acceptability of the daily enhancement of meaningful activity program: Intervention for mild cognitive impairment patient-spouse dyads. J Neurosci Nurs. 2011;43(6):317–328. doi: 10.1097/JNN.0b013e318234e9dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu YY, Bakas T, Haase JE. Cost template for meaningful activity intervention for mild cognitive impairment. Clin Nurse Spec. 2013;27(2):88–95. doi: 10.1097/NUR.0b013e3182819171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kroenke K, Spitzer RL, Williams JB. The phq-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO. World Health Organization. International classification of functioning, disability and health. Geneva: World Health Organ; 2001. [Google Scholar]
- 16.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40(9):771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Nowotny ML. Assessment of hope in patients with cancer: Development of an instrument. Oncol Nurs Forum. 1989;16(1):57–61. [PubMed] [Google Scholar]
- 19.McColl MA, Paterson M, Davies D, Doubt L, Law M. Validity and community utility of the Canadian occupational performance measure. Can J Occup Ther. 2000;67(1):22–30. doi: 10.1177/000841740006700105. [DOI] [PubMed] [Google Scholar]
- 20.Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s disease cooperative study. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S33–39. [PubMed] [Google Scholar]
- 21.Quinn C, Toms G, Anderson D, Clare L. A review of self-management interventions for people with dementia and mild cognitive impairment. J Appl Gerontol. 2015 doi: 10.1177/0733464814566852. (Epub Jan 21) [DOI] [PubMed] [Google Scholar]
- 22.NIA. Global health and aging. 11-7737. Washington, DC: NIH; 2011. [Google Scholar]
- 23.Curtis RG, Huxhold O, Windsor TD. Perceived control and social activity in midlife and older age: A reciprocal association? Findings from the German ageing survey. J Gerontol B Psychol Sci Soc Sci. 2016:71. doi: 10.1093/geronb/gbw070. (Epub June 17) [DOI] [PubMed] [Google Scholar]
- 24.O’Shea DM, Dotson VM, Fieo RA, Tsapanou A, Zahodne L, Stern Y. Older adults with poor self-rated memory have less depressive symptoms and better memory performance when perceived self-efficacy is high. Int J Geriatr Psychiatry. 2016;31(7):783–790. doi: 10.1002/gps.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomioka K, Kurumatani N, Hosoi H. Social participation and cognitive decline among community-dwelling older adults: A community-based longitudinal study. J Gerontol B Psychol Sci Soc Sci (Abstract) 2016 doi: 10.1093/geronb/gbw059. [DOI] [PubMed] [Google Scholar]
- 26.Tomioka K, Kurumatani N, Hosoi H. Association between social participation and instrumental activities of daily living among community-dwelling older adults. J Epidemiol. 2016 doi: 10.2188/jea.JE20150253. (Epub May 14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vellone E, Pancani L, Greco A, Steca P, Riegel B. Self-care confidence may be more important than cognition to influence self-care behaviors in adults with heart failure: Testing a mediation model. Int J Nurs Stud. 2016;60:191–199. doi: 10.1016/j.ijnurstu.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Cene CW, Haymore LB, Dolan-Soto D, et al. Self-care confidence mediates the relationship between perceived social support and self-care maintenance in adults with heart failure. J Card Fail. 2013;19(3):202–210. doi: 10.1016/j.cardfail.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levasseur M, Desrosiers J, St-Cyr Tribble D. Do quality of life, participation and environment of older adults differ according to level of activity? Health Qual Life Outcomes. 2008;6:30. doi: 10.1186/1477-7525-6-30. (Epub April 14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarzbach M, Luppa M, Forstmeier S, Konig HH, Riedel-Heller SG. Social relations and depression in late life-a systematic review. Int J Geriatr Psychiatry. 2014;29(1):1–21. doi: 10.1002/gps.3971. [DOI] [PubMed] [Google Scholar]
- 31.Reppermund S, Sachdev PS, Crawford J, et al. The relationship of neuropsychological function to instrumental activities of daily living in mild cognitive impairment. Int J Geriatr Psychiatry. 2011;26(8):843–852. doi: 10.1002/gps.2612. [DOI] [PubMed] [Google Scholar]
- 32.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barber SE, Clegg AP, Young JB. Is there a role for physical activity in preventing cognitive decline in people with mild cognitive impairment? Age Ageing. 2012;41(1):5–8. doi: 10.1093/ageing/afr138. [DOI] [PubMed] [Google Scholar]
- 34.HHS; [Accessed 01/25/2016];Services USDoHaH, editor. National plan to address Alzheimer’s disease: 2013 update. 2015 Retrieved from https://aspe.hhs.gov/sites/default/files/pdf/107031/natlplan2015.pdf.
- 35.Lu YY, Ellis J, Yang Z, et al. Satisfaction with a family-focused intervention for mild cognitive impairment dyads. J Nurs Scholarsh. 2016;48(4):334–344. doi: 10.1111/jnu.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]