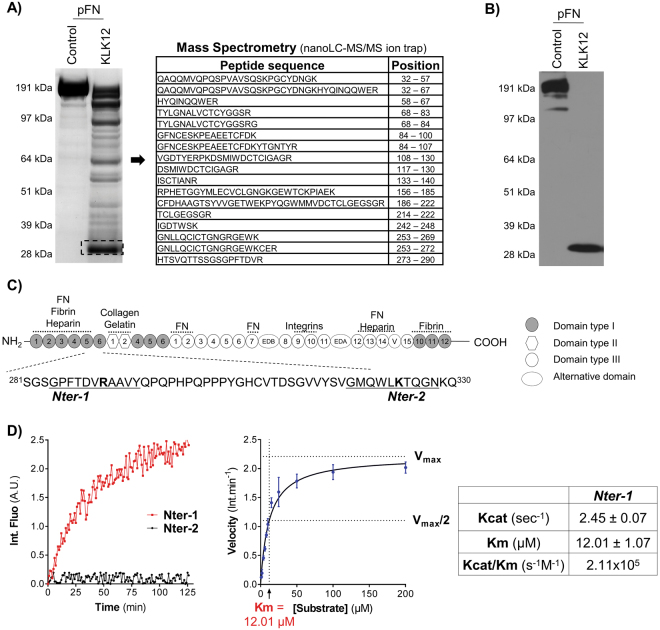
Figure 2.
KLK12 releases the amino-terminal domains of fibronectin. (A) Characterization of the 29 kDa proteolytic fragment of FN (FN-f) generated by KLK12. After proteolysis of pFN by KLK12 (E:S molar ratio: 1:50; 4 h at 37 °C), FN proteolytic fragments were separated by SDS-PAGE, and the 29 kDa fragment was analyzed using mass spectrometry. The sequences and position of peptides corresponding to the amino-terminal domains of FN are indicated. MS/MS results are summarized in Supp Table 1. (B) Western blot of FN proteolytic fragments generated by KLK12 (as in A), using an FN9-1 antibody directed against the amino-terminal domains of FN. Western blots with other anti-FN antibodies are shown in Supp. Figure 1. (C) A schematic depiction of the domain structure of FN. Intra- and intermolecular interactions are indicated above the diagram. The sequences of the two potential KLK12-cleavage sites (leading to the release of the 29-kDa fragment) are indicated below the diagram (Nter-1 and Nter-2). (D) Left panel: hydrolysis of Nter-1 and Nter-2 FRET peptides (0.5 µM) by KLK12 (7 nM). Right panel: non-linear regression curve for hydrolysis of the Nter-1 peptide by KLK12. The initial velocity (in fluorescence intensity per minute (Int.min−1)) of the E:S reaction was measured with increasing S concentrations (0–200 μM). Table: Kinetic constants calculated for the Nter-1 peptide in a non-linear regression analysis.