Abstract
Secretory immunoglobulin (Ig) A is a decameric Ig composed of four α-heavy chains, four light chains, a joining (J) chain, and a secretory component (SC). The heavy and light chains form two tetrameric Ig molecules that are joined by the J chain and associate with the SC. Expression of a secretory monoclonal antibody in tobacco (Nicotiana tabacum) has been described: this molecule (secretory IgA/G [SIgA/G]) was modified by having a hybrid heavy chain sequence consisting of IgG γ-chain domains linked to constant region domains of an IgA α-chain. In tobacco, about 70% of the protein assembles to its final, decameric structure. We show here that SIgA/G assembly and secretion are slow, with only approximately 10% of the newly synthesized molecules being secreted after 24 h and the bulk probably remaining in the endoplasmic reticulum. In addition, a proportion of SIgA/G is delivered to the vacuole as at least partially assembled molecules by a process that is blocked by the membrane traffic inhibitor brefeldin A. Neither the SC nor the J chain are responsible for vacuolar delivery, because IgA/G tetramers have the same fate. The parent IgG tetrameric molecule, containing wild-type γ-heavy chains, is instead secreted rapidly and efficiently. This strongly suggests that intracellular retention and vacuolar delivery of IgA/G is due to the α-domains present in the hybrid α/γ-heavy chains and indicates that the plant secretory system may partially deliver to the vacuole recombinant proteins expected to be secreted.
The secretory pathway delivers proteins from the endoplasmic reticulum (ER), where secretory proteins are cotranslationally inserted, to the cell surface or the inner hydrolytic compartments (vacuoles in plants and yeast, or lysosomes in animals). This traffic is often mediated by the Golgi complex. A vast array of proteins of pharmaceutical importance are secreted by different mammalian cells, and transgenic plants are an attractive expression system for producing recombinant forms of these proteins. One of the major advantages is that many steps of the secretory pathway, including protein folding, assembly, ER-mediated glycosylation, and early steps of Golgi-mediated glycan processing, are largely similar, when not identical, to those found in mammalian cells (Ma and Hein, 1995; Rayon et al., 1998; Sanderfoot and Raikhel, 1999; Vitale and Denecke, 1999). Thus mammalian-secreted proteins can be produced with a high degree of fidelity. One such example is murine immunoglobulin (Ig) G monoclonal antibody. This molecule comprises four polypeptides—two each of a heavy and a light chain, which are linked by disulfide bonds. In mammalian plasma cells, correct assembly of this molecule is achieved within the ER through interactions with a number of chaperones and enzymes and the addition of glycans prior to secretion from the cell. These events are faithfully reproduced in plant cells, to the extent that IgG can be expressed that is functionally indistinguishable from the same antibody expressed in murine cells and is expressed at levels of 1% of total soluble leaf protein (Hiatt et al., 1989; Ma et al., 1995).
When a related but more complex Ig, a recombinant secretory Ig A/G (SIgA/G) hybrid, was expressed in plants, the levels of accumulation were even greater, amounting to 5% to 8% of total soluble leaf protein (Ma et al., 1995). Secretory IgA (SIgA) is a decameric polypeptide complex. In the ER of plasma cells, two standard Ig units (each composed of two heavy and two light chains, like IgG) are first dimerized by a joining (J) chain to form dimeric IgA (dIgA), held by a disulfide bond between each tetramer and the J chain. In mammals, after secretion from plasma cells, dIgA is recognized by a receptor present on the basolateral surface of epithelial cells. Transcytosis causes transport of the receptor/ligand complex to the apical surface, where a proteolytic event releases the dIgA associated to a portion of the receptor (called secretory component [SC]), resulting in the formation of the full, decameric SIgA molecule (Mestecky and McGhee, 1987). Thus, in its natural environment SIgA is the result of the activities of two cell types, but in transgenic plants it is successfully synthesized in the ER of individual cells. One of the roles of the SC is to protect the secretory Ig against proteolysis in vivo (Underdown and Dorrington, 1974). Thus, SIgA is a more stable molecule than IgG, however it is unlikely that this alone would account for the difference in accumulation levels between SIgA/G and IgG in plants. In this study we compared the fates of the IgG and SIgA/G in plant cells to determine if there were any differences in the way plants handle these proteins.
One of the peculiar characteristics of the plant secretory pathway is the presence of vacuoles. Although yeast has vacuoles as well and mammals have lysosomes (rich in hydrolases, similar to lytic plant vacuoles), the ontogeny, variety of functions, and protein sorting mechanisms of plant vacuoles are unique (Neuhaus and Rogers, 1998; Marty, 1999). We report that after entry into the plant secretory pathway, a relevant proportion of SIgA/G, but not of IgG, is diverted from secretion and eventually delivered as at least partially assembled molecules to the central vacuole of tobacco (Nicotiana tabacum) leaf mesophyll cells. This process is sensitive to the membrane traffic inhibitor brefeldin A. Thus, plant cells may deliver to the vacuole a proportion of mammalian recombinant proteins that are expected to be secreted.
RESULTS
Secretion of SIgA/G Is Slow
In SIgA/G, the heavy chains are hybrids containing the variable domains and the constant Cγ1 and Cγ2 domains of the IgG γ-chain linked to the constant Cα2 and Cα3 domains of the IgA α-chain. These extra domains allow the Ig units (composed of two heavy and two light chains) to dimerize by binding the J chain and to further associate with the SC (Ma et al., 1995).
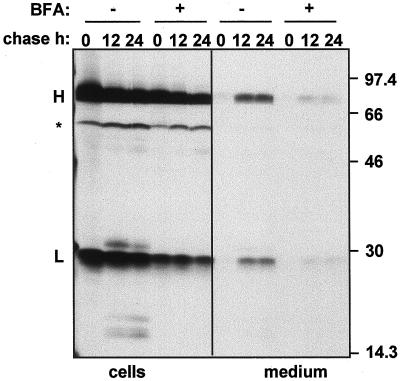
We first wanted to establish the efficiency of SIgA/G secretion. We isolated protoplasts from leaves of SIgA/G-expressing tobacco plants and subjected them to pulse-chase analysis. We then immunoprecipitated the whole SIgA/G molecule from cell lysates and incubation media using polyclonal anti-IgG antiserum, and analyzed the polypeptides on reducing SDS-PAGE and fluorography. Figure 1 shows that, at the end of the pulse period, the heavy IgA/G chain, and the light chain, together with a polypeptide of the expected size for the un-glycosylated SC are detected. Glycosylated SC comigrates with the heavy chain, and the J chain, which normally forms dimers on reducing SDS-PAGE, comigrates with the light chain (F. Wang and M.B. Hein, unpublished results). After 12 h, only a small proportion (about 10%) of the antibody is retrieved from the medium; the amount of secreted protein increases only slightly in the following 12 h, whereas the bulk of the antibody remains intracellular. At 5 h of chase the amount of secreted IgA/G is below our detection limit (not shown). Thus, secretion of SIgA/G is a very slow and apparently inefficient process. This seems to be close to the bottom limit of a range observed for heterologous proteins introduced into the plant secretory pathway (Denecke et al., 1990), but a variety of wild-type and recombinant proteins are secreted in a much shorter time, with half-times within 2 to 5 h from the time of synthesis (Hunt and Chrispeels, 1991; Matsuoka and Nakamura, 1991; Frigerio et al., 1998). When protoplasts were incubated in the presence of 10 μg mL−1 brefeldin A, secretion was greatly reduced (Fig. 1). This concentration of brefeldin A has been shown to inhibit membrane traffic from the ER (Boevink et al., 1998).
Figure 1.
SIgA/G is secreted slowly. Protoplasts from leaves of transgenic tobacco expressing SIgA/G were pulse-labeled for 1 h with [35S]Met and [35S]Cys either in the presence (+) or in the absence (−) of brefeldin A (BFA) and chased for the indicated periods of time. Cells and the corresponding incubation media were homogenized, subjected to immunoprecipitation with anti-IgG antiserum, and analyzed by 15% (w/v) reducing SDS-PAGE and fluorography. Numbers at right indicate molecular mass markers in kD. H, Heavy chain; L, light chain; asterisk, un-glycosylated SC.
After 12 h of chase, several discrete polypeptides in the 15- to 30-kD range were detected in the protoplast lysates (Fig. 1, no brefeldin A treatment). The presence of intracellular Ig breakdown products was previously reported (Ma et al., 1994, 1995). Their absence at the end of the pulse and appearance during the chase rule out the possibility that these fragments were generated during sample homogenation. The appearance of these fragments was efficiently inhibited by brefeldin A treatment (Fig. 1; 12- and 24-h chase in the presence of brefeldin A), indicating that fragmentation occurs because of transit to a post-ER compartment through membrane traffic (see further below).
The Intracellular Breakdown Products Are Located in the Vacuole
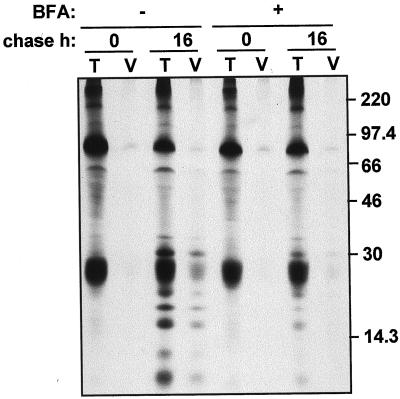
We wanted to investigate where the Ig breakdown products are located. The fact that degradation can be prevented by brefeldin A treatment, which inhibits delivery to plant vacuoles (Gomez and Chrispeels, 1993), suggested that the vacuole could be the site of degradation. To test this hypothesis, we subjected SIgA/G-expressing protoplasts to pulse-chase either in the presence or in the absence of brefeldin A. We then purified vacuoles from protoplasts and immunoselected the Ig (Fig. 2). After 16 h of chase the Ig fragmentation products were clearly detectable in the vacuolar fraction; consistently, treatment with brefeldin A prevented the appearance of the fragments in the vacuoles. We then conclude that a proportion of the SIgA/G molecules is diverted to the vacuole by membrane traffic. Intact light chains also seem to be in part located in the vacuoles; whereas the extremely low proportion of intact heavy chains in the vacuolar fraction is most likely a slight contamination from another cellular compartment, because it is also detectable, at similar levels, at the end of the pulse-labeling and is not affected by brefeldin A treatment.
Figure 2.
A proportion of SIgA/G is targeted to the vacuole and fragmented. Protoplasts from SIgA/G-expressing plants were pulse-labeled for 1 h either in the presence (+) or in the absence (−) of brefeldin A and chased for the indicated periods of time. Total cell homogenates (T) or purified vacuoles (V) were then subjected to immunoprecipitation with anti-IgG antiserum and analyzed by 15% (w/v) reducing SDS-PAGE and fluorography. Numbers at right indicate molecular mass markers in kD.
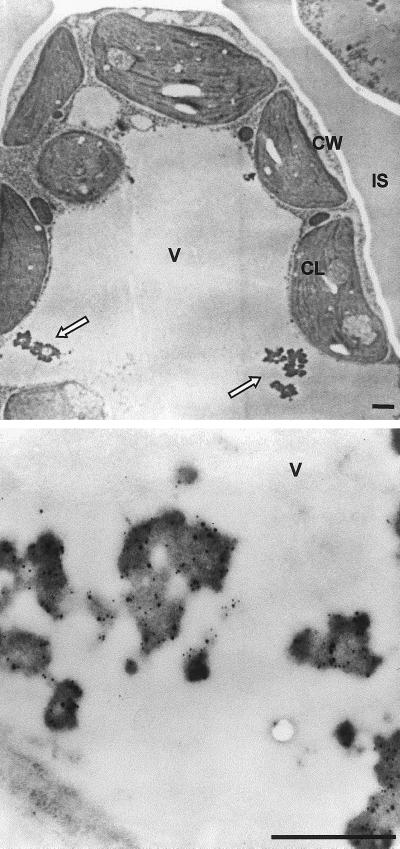
Immunoelectron microscopy indicated that the steady-state level of recombinant protein present in vacuoles was not irrelevant. In sections of leaves from SIgA/G-expressing plants, gold-conjugated anti-γ-chain (small particles) decorated large vacuolar aggregates, which were not detectable in wild-type plants (Fig. 3). These aggregates closely resemble protein body-like structures, which have been observed when vacuolar proteins (such as barley lectin and bean phaseolin) were expressed in leaf cells (Dombrowski et al., 1993; Frigerio et al., 1998). The anti-SC antibody (large particles) decorated the same protein bodies as the anti-γ chain.
Figure 3.
SIgA/G forms protein body-like aggregates in the vacuole. Ultra-thin sections of transgenic tobacco leaves expressing SIgA/G (top panel) were incubated with anti-SC and anti-Ig antibodies (bottom panel). Detection was performed using secondary antibodies conjugated with 12 nm (for SC) and 6 nm (for Ig) colloidal gold. Arrows indicate the vacuolar protein body-like structures. CL, Chloroplast; CW, cell wall; IS, intercellular space; V, vacuole. Bars = 1 μm.
Vacuolar Delivery Is Caused by Sequences in the IgA/G Tetramer
The finding that a percentage of antibody in the SIgA/G plants is not secreted but rerouted to the vacuole, raises the question of whether this vacuolar targeting is the product of a positive signaling by the molecule, or rather represents an escape route for the plant endomembrane system when overloaded with foreign molecules.
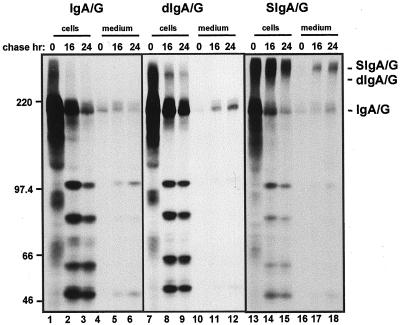
We first determined whether vacuolar sorting could be caused by one or more components of the SIgA/G molecule, namely the SC or the J chain. Therefore we analyzed transgenic plants expressing either the sole IgA/G tetrameric unit (IgA/G) or the J-chain linked IgA/G dimer (dIgA/G) and compared the fate of these assembly intermediates to that of the whole SIgA/G molecule. We subjected protoplasts from leaves of the indicated transgenic plants to pulse-chase and immunoprecipitation with anti-IgG antiserum. We then analyzed the polypeptides by non-reducing, 6% (w/v) acrylamide SDS PAGE and fluorography (Fig. 4) to establish the level of assembly of the Ig molecules. In non-reducing conditions the vacuolar fragments originating from SIgA/G migrate in the 45- to 100-kD range (Fig. 4, lanes 14 and 15). This indicates that the fragments are not fully unassembled or disassembled components of the Ig molecule. The observation that the majority of the polypeptides migrate under non-reducing conditions as tetrameric IgA/G units after 1 h of pulse (Fig. 4, lane 13) indicates fast and efficient assembly of the tetramers.
Figure 4.
Vacuolar targeting and slow secretion are independent of the assembly state of SIgA/G. Protoplasts from leaves of transgenic tobacco plants expressing IgA/G, dIgA/G, or SIgA/G were pulse-labeled for 1 h and chased for the indicated periods of time. Cells and the corresponding incubation media were homogenated, subjected to immunoprecipitation with anti-IgG antiserum, and analyzed by 6% (w/v) non-reducing SDS-PAGE and fluorography. Numbers at left indicate molecular mass markers in kD.
Clearly, we observed the presence of identical major fragmentation products in all cell types, including the ones expressing the tetrameric IgA/G unit only (Fig. 4, compare lanes 2 and 3, 8 and 9, 14 and 15). This indicates that delivery to the vacuole does not require the J chain or the SC. The ratio of intact to fragmented molecules was higher in SIgA/G-expressing plants (Fig. 4, lanes 13–18), indicating that either the fully assembled SIgA/G is more resistant to proteolysis in the vacuole or the SC and the J chain partially inhibit vacuolar delivery of IgA/G. The IgA/G unit seemed to be partially degraded even after secretion in the incubation medium (Fig. 4, lanes 5 and 6). It has been reported that proteins secreted by tobacco protoplasts (Frigerio et al., 1998) and cultured cells (Matsuoka et al., 1995) may undergo proteolysis. Again, secretion of SIgA/G was slow and inefficient (Fig. 4, lanes 16–18, and see Fig. 1, lanes 7–9). A similar rate of secretion was observed for the “monomeric” form of the antibody both in IgA/G and dIgA/G plants. No secretion of the dimeric molecule was detected, because either the dimers are unavailable for secretion for unknown reasons or the J chain is particularly susceptible to extracellular proteolysis when not protected by the SC.
The Parent IgG Is Rapidly and Efficiently Secreted
The results shown so far indicate that, even in their simplest, tetrameric form, plant-made IgA/Gs are inefficiently secreted and in part delivered to the vacuole. Is intracellular retention due to domains in the IgA/G molecule or is it due to a more general problem, as the plant secretory system must deal with the synthesis and assembly of complex, bulky molecules such as Igs? If the latter is true, we would expect the parent murine IgG molecule to undergo the same fate as its IgA/G derivative. Indeed, the substitution of the native Cγ3 domain with Cα2 and Cα3 domains from an IgA-secreting hybridoma has no negative effect on the assembly and activity of the hybrid molecule (Ma et al., 1995, 1998) and the sequence of assembly events is virtually identical.
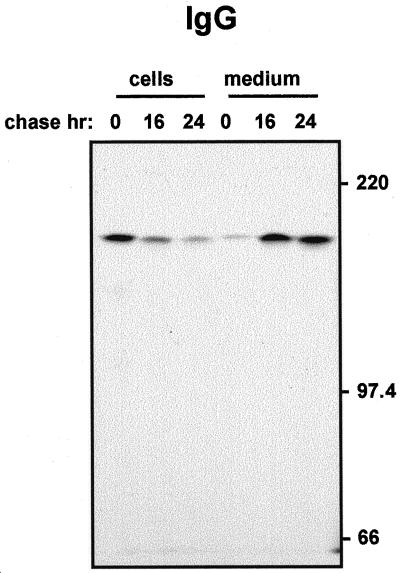
To test this, we isolated protoplasts from leaves of Guy's 13 IgG producing plants and followed the fate of the IgG molecule by pulse-chase (Fig. 5). We found that almost all the IgG synthesized during the pulse was recovered after 24 h of chase from the protoplast incubation medium. The secreted IgG was tetrameric and intact, and no intracellular degradation products were detectable (Fig. 5). Thus, secretion was nearly quantitative. Moreover, the rate of secretion was much faster than that of SIgA/G: About 70% of the molecules were secreted after 16 h.
Figure 5.
The parent IgG molecule is secreted efficiently. Protoplasts from leaves of transgenic tobacco plants expressing the monoclonal Guy's 13 IgG were pulse-labeled for 1 h and chased for the indicated periods of time. Cells and the corresponding incubation media were homogenized, subjected to immunoprecipitation with anti-IgG antiserum, and analyzed by 6% (w/v) non-reducing SDS-PAGE and fluorography. Numbers at right indicate molecular mass markers in kD.
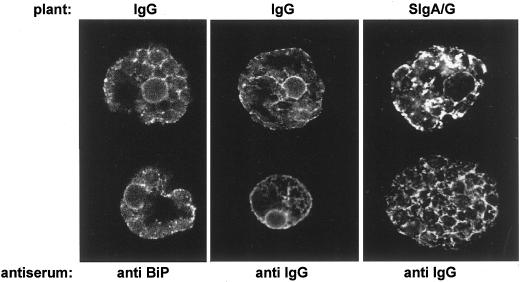
The difference in the fate of IgG compared to that of SIgA/G is also evident at steady state (Fig. 6). We detected the Ig molecules by immunofluorescence confocal laser scanning microscopy on fixed, permeabilized protoplasts from IgG or SIgA/G transgenic plants. The anti-IgG antiserum did not stain any structure in cells prepared from un-transformed tobacco (not shown). In the case of IgG-producing cells (Fig. 6, center panel), the steady-state amount of Ig detected in the secretory system represents only the newly synthesized molecules and the molecules en route to secretion, as testified by the relevant proportion of staining in proximity of the plasma membrane. This was confirmed by comparison with the distribution of the ER resident chaperone BiP (Fig. 6, left panel). Whereas the perinuclear and reticular structures were similarly stained, staining in proximity of the plasma membrane was much less intense. It is also evident that the endomembrane system of SIgA/G producing plants is literally laden with antibody molecules (Fig. 6, right panel). The reticular structure is similar to the one detectable using antibodies against the BiP, but in the case of SIgA/G its appearance is more swollen and punctate structures, possibly representing vacuolar depositions, are often detectable as well.
Figure 6.
The bulk of SIgA/G is retained in the endomembrane system. Protoplasts from either IgG- or SIgA/G-expressing plants were fixed, permeabilized, and subjected to immunofluorescence with primary rabbit anti-IgG serum or anti-BiP serum, followed by secondary fluorescein isothiocyanate-conjugated goat anti-rabbit antibody. Cells were observed with a confocal laser scanning microscope at 494-nm excitation and 522-nm emission wavelength. For IgG cells, laser intensity was 10%, gain 1,200; for SIgA/G cells, laser intensity was 3%, gain 950.
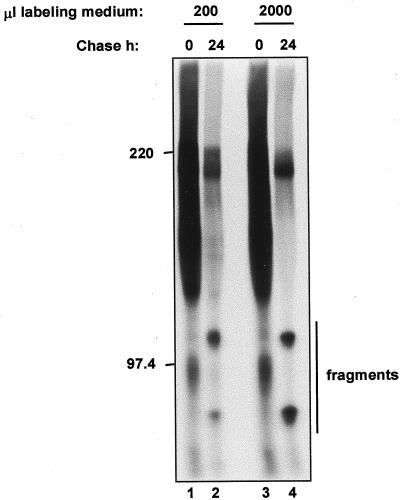
Vacuolar delivery could in theory result from endocytosis of secreted molecules. However, the results shown in Figure 5 indicate that secreted IgGs are certainly not subjected to endocytosis to a relevant extent, ruling out unspecific uptake of protein from the medium. Moreover, when incubation during the chase was performed under agitation in a 10-fold excess of incubation medium with respect to our standard protocol, there was no reduction in the efficiency of IgA/G fragmentation (Fig. 7). Therefore, most probably vacuolar IgA/G is not a fraction of secreted molecules that have been endocytosed.
Figure 7.
IgA/G fragmentation is not due to endocytosis. Protoplasts from plants expressing IgA/G were pulse-labeled for 1 h in the indicated volumes of incubation medium and chased for the indicated periods of time. Cells were then homogenated and subjected to immunoprecipitation with anti-IgG antiserum, followed by 6% (w/v) non-reducing SDS-PAGE and fluorography. Numbers on the left indicate molecular mass markers in kD. Only the intact protein and the higher molecular mass fragments are shown.
Newly Synthesized IgA/G Is a Soluble Protein
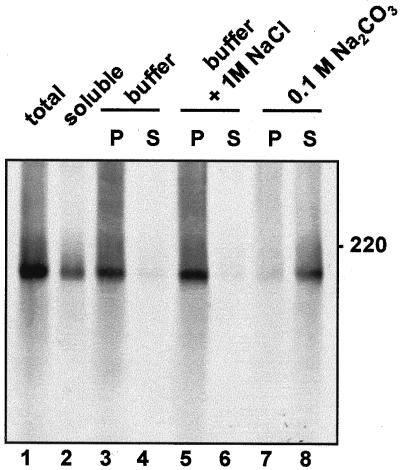
In mammalian cells, proteins that are anchored to the ER membrane follow the secretory pathway until they reach the plasma membrane, unless they have specific features that allow their ER retention or sorting to other membranes of the endomembrane system (Pedrazzini et al., 1996). The question of a default location for membrane proteins is still open for plant cells, and the tonoplast is a possible candidate (Barrieu and Chrispeels, 1999). In addition the newly synthesized precursor of the soluble vacuolar storage protein legumin is tightly associated to the lumenal side of ER/Golgi membranes and its membrane binding is unaffected by agents that usually release peripheral membrane proteins (Hinz et al., 1997). It has been suggested that high affinity to membranes may have a role in the vacuolar sorting of legumin and maybe other storage proteins (Saalbach et al., 1991). We therefore verified whether newly synthesized IgA/G is tightly associated to the endomembranes. Protoplasts from transgenic leaves were pulse-labeled for 1 h and homogenated in the absence of detergent and the presence of Suc. The homogenate was fractionated into a microsomal and a soluble fraction. The latter contains cytosolic proteins and the content of vacuoles, which break during homogenation under these conditions. Consistently, newly synthesized IgA/G is mainly recovered in the microsomal fraction (Fig. 8, lanes 1 and 2). Microsomes were then washed with Suc buffer as a control, with 1 m sodium chloride in Suc buffer, or with 0.1 m sodium carbonate. Sodium carbonate releases soluble proteins present in the lumen of microsomes but not integral membrane proteins (Fujiki et al., 1982), whereas sodium chloride releases proteins peripherally attached to the cytosolic face of microsomes without affecting their lumenal content. As expected for a soluble lumenal protein that is not tightly bound to the membrane, IgA/G is released by sodium carbonate but not by sodium chloride (Fig. 8, lanes 3–8). We conclude that tight association to the endomembranes cannot explain the vacuolar delivery of IgA/G.
Figure 8.
IgA/G does not associate tightly with membranes. Protoplasts from plants expressing IgA/G were pulse-labeled for 1 h and homogenized in 12% (w/w) Suc buffer. Microsomes were prepared and resuspended in Suc buffer, in Suc buffer with 1 m NaCl, or in 0.1 m Na2CO3, and incubated on ice for 30 min. Microsomes were then reloaded on top of a 17% (w/w) Suc pad and centrifuged for 30 min at 150,000g. Supernatants (S) and pellets (P) were homogenated, immunoprecipitated with anti-IgG antiserum, and analyzed by 6% (w/v) non-reducing SDS-PAGE and fluorography. total, Anti-IgG immunoprecipitate from total cell homogenate. soluble, Anti-IgG immunoprecipitate from the supernatant from the first microsome preparation, containing cytosolic and vacuolar proteins.
DISCUSSION
In this paper we show that secretion of SIgA/G proceeds at a very slow rate in tobacco leaf cells. Even 24 h after synthesis, the vast majority of molecules has not been secreted. Moreover, part of the protein is transported to the vacuole where it is detectable as fragmentation products. In contrast the parent IgG molecules are quantitatively secreted with a half-time of less than 16 h. This raises the problem of which are the mechanisms and recognition events that lead to vacuolar delivery of a protein that is expected to be secreted. Besides the implications for the production of IgA in plants, this can shed light on still unknown features of the plant secretory pathway which may have general relevance for both plant metabolism and the use of plants to produce heterologous secretory proteins.
Slow and Inefficient Secretion
Our experiments rule out the possibility that the J chain and the SC are responsible for the slow secretion and for vacuolar delivery of SIgA/G. Therefore, the modification of the heavy chain by deletion of the Cγ3 domain and the addition of Cα2 and Cα3 domains (Ma et al., 1994) is the structural characteristic that leads to the altered secretory phenotype.
The rates of secretion from tobacco protoplasts of three bacterial proteins introduced into the secretory pathway have been compared previously (Denecke et al., 1990). Although the assay used to measure secretion was different from ours, the results pointed to a high degree of variability in secretion efficiency of heterologous proteins in plant cells. An inverse correlation was found in that case between secretory rate and size of the passenger protein, but the reasons for such variability remained unknown.
Secretion rate and efficiency can certainly be determined by ER quality control. This mechanism assists the folding and assembly of newly synthesized proteins, in most cases allows their traffic along the secretory pathway only upon correct completion of these maturation events and eventually targets defective polypeptides for degradation (Vitale and Denecke, 1999). Confocal microscopy suggested that a large fraction of SigA/G is indeed present in the ER. Analysis of its oligomerization state by SDS-PAGE in non-reducing conditions showed that after 1-h pulse, there were no free heavy or light chains, most of the molecules assembled into tetrameric IgA/G units, and fully assembled SigA/G were already detectable. The proportion of assembled SigA/G increased markedly after 16 h of chase, to represent more than 60% of the total immunoprecipitable polypeptides. This indicates that the rate and efficiency of heterotetramer formation are high, comparable to those of the bean homotrimeric protein phaseolin expressed in tobacco leaves (Frigerio et al., 1998), and that inefficient assembly is unlikely to be the cause of ER retention of IgA/G.
In mammalian cells, exposed Cys residues in the C-terminal region of the heavy chains of IgA and secretory IgM are recognized by ER quality control; as a result, in B lymphocytes tetramers are retained in this compartment (Sitia et al., 1990; Guenzi et al., 1994). The efficiency of this retention is both dependent on the stage of B cell development and the amino acid context surrounding the Cys. In plasma cells, IgA but not IgM are secreted, albeit slowly, and the different behavior of the two Igs is due to the presence of an extra acidic residue upstream of the Cys in IgA but not IgM (Guenzi et al., 1994). On the other hand, mutation of the critical Cys results in very efficient secretion of IgM monomers (Sitia et al., 1990). Thus, in mammalian cells thiol-mediated retention is responsible for the decreasing rates of secretion of IgG, IgA, and secretory IgM tetramers, respectively. Our observation that in tobacco the parent IgG tetramers, which do not have free cysteines, are secreted with high efficiency is consistent with the possibility that thiol-mediated retention also occurs in the plant ER. This hypothesis can be tested by mutagenesis or in vivo treatment with reducing agents.
We cannot rule out the alternative possibility that, in spite of correct assembly and independently of the exposed Cys residues, some of the tetramers or decamers have conformational defects that lead to their prolonged ER retention and eventual slow degradation. The IgA/G heavy chain is not a naturally synthesized molecule—it contains a mixture of γ- and α-chain domains, as well as an extra Cα2 domain. Although this might affect the dimerization of heavy chains, it seems unlikely that this modification would affect assembly with Ig light chain, or even J chain and SC. However, we have no experience of how addition of an extra Cα2 domain in the heavy chain might affect interactions with chaperones or recognition by quality control mechanisms in the ER.
Delivery to the Vacuole
Fragmentation of a proportion of the IgA/G (monomer, dimer, or secretory) molecules occurs either soon before or upon delivery to the vacuole: Fragments are detected in vacuoles. This vacuolar delivery is inhibited by brefeldin A, indicating active vesicular transport out of the ER along the secretory pathway.
Plant cells, like mammalian cells, have a default route that delivers out of the cell proteins that are inserted into the ER (Denecke et al., 1990). To be delivered to the vacuole, soluble proteins need sorting signals; when these signals are deleted, the mutated proteins are secreted, albeit with variable efficiencies (Bednarek et al., 1990; Crofts et al., 1999). Although one potential receptor for vacuolar sorting has been identified, the mechanisms for vacuolar delivery are not yet fully clarified, and certainly more than one mechanism exists (Vitale and Raikhel, 1999). It has been hypothesized that aggregation and high affinity to membranes can be the mechanism that sorts some storage proteins to vacuoles (Saalbach et al., 1991; Vitale and Raikhel, 1999). However we were unable to demonstrate tight binding of IgA/G to endomembranes. Endocytosis followed by vacuolar delivery also seems an unlikely possibility in the light of our results.
We are not aware of previous reports of vacuolar delivery of a protein expected to be secreted in plant cells, but other proteins that do not reside in the vacuole in their natural cells are in part delivered to vacuoles when expressed in transgenic plants. These are the maize zeins, which in maize accumulate as ER-located protein bodies but are partially found in vacuoles in transgenic tobacco (Coleman et al., 1996). The recognition events that lead to this mis-localization have not been established, but autophagy has been proposed as a possible explanation (Coleman et al., 1996). It is not known whether this process can be inhibited by brefeldin A.
In mammalian cells, prolonged ER retention of un-polymerized IgA and IgM tetramers or certain unassembled mutant light chains can result in quality control degradation. Degradation of unassembled light chains, IgM and J chains is sensitive to proteasome inhibitors, strongly suggesting that it occurs upon dislocation of the polypeptides from the ER into the cytosol (Chillaron and Haas, 2000; Mancini et al., 2000; C. Fagioli and R. Sitia, personal communication), a fate similar to the one of several other mammalian and yeast proteins subjected to ER associated degradation (Brodsky and McCracken, 1999). Therefore, if quality control is responsible for the fragmentation of IgA/G in tobacco, the subcellular location of the hydrolytic activity is different with respect to the one identified in mammalian cells. In yeast cells, it has been shown that defective proteins can be delivered for degradation to the vacuole through the Golgi complex (Hong et al., 1996); again the recognition mechanism is not clear but this has been suggested to be a quality control disposal route alternative to retrotranslocation from the ER in the cytosol, which conversely does not involve vesicular traffic. Such a Golgi-mediated route may be active also in plant cells and take care of the degradation of a proportion of antibody molecules with exposed Cys residues or defects that could be not be detected by our assays.
The polypeptides targeted to the vacuole and fragmented are, at least in part, assembled, although we have not yet determined their exact assembly status. In the light of the analysis of the oligomerization state after pulse-labeling, the most reasonable hypothesis is that the major Ig form delivered to the vacuole is the tetrameric unit. A proportion of these units would be delivered to the vacuole after prolonged ER retention. The colocalization of the heavy chain and the SC in the same vacuolar aggregates, detectable by immunoelectron microscopy, does not necessarily imply that the two molecules are part of the same assembled antibody: when two noninteracting vacuolar proteins were co-expressed in transgenic plants they colocalized in vacuolar aggregates as well (Schroeder et al., 1993). The proportion of SC found in the vacuole could in this case be delivered to this compartment in different forms: associated to intermediates of assembly or as individual, unassembled molecules, whereas fully assembled SIgA/G would be secreted.
An alternative hypothesis with respect to quality control delivery to the vacuole could be that saturation of secretion, due to high levels of Ig synthesis, leads to delivery of the excess of protein to the vacuole, independently of the presence of the J chain and SC. We do not favor this hypothesis, because of the behavior of IgG and of the much higher proportion of fragmented IgA/G molecules generated in the absence of the J chain and the SC, but it cannot be conclusively ruled out. The opposite is certainly true: Overexpression of vacuolar proteins leads to their partial Golgi-mediated secretion (Frigerio et al., 1998). Vacuolar delivery upon high expression would imply that secretion from plant cells is a saturable process. Such a scenario would be against the current model that secretion is the default route for proteins inserted into the secretory pathway. There is very solid evidence that a default route to secretion exists, but several observations suggest that active selection of cargo proteins destined for secretion may also occur (Vitale and Denecke, 1999). The two mechanisms, default and active selection, might co-exist, the latter being saturable and its saturation leading to vacuolar delivery as an alternative route. This hypothesis can be tested by co-expressing with SIgA/G a protein known to be secreted by plant cells with high efficiency.
Finally, irrespective of the quality control or saturation hypothesis, our observation that vacuolar fragmentation is higher in the absence of the SC, also when the J chain that allows dimerization is synthesized, indicates an active role for the SC in promoting secretion and/or protecting from degradation in the vacuole. We believe this is important. It should be remembered that in the natural organism where SIgA are produced, addition of the SC is an extracellular event. Clearly, this component is also able to alter the destiny of Ig molecules within the plant endomembrane system.
Localization and Stability
SIgA/G and the parent IgG accumulate in tobacco leaves to 5% to 8% and 1% of total soluble proteins, respectively (Ma et al., 1994, 1995). What is the reason of this difference? We originally hypothesized that the SC might protect SIgA/G from degradation in the apoplast (Ma et al., 1995). The results presented here suggest other possible explanations.
Certainly, a proportion of SIgA/G is present in the ER and in the vacuole. The ER has been shown to be a very safe compartment for recombinant secretory proteins expressed in transgenic plants: when proteins were retained in the ER via the addition of the KDEL signal they showed markedly increased stability (Wandelt et al., 1992; Pueyo et al., 1995; Tabe et al., 1995; Conrad and Fiedler, 1998). ER retention of SIgA/G is certainly not due to the presence of a known ER retention signal, and might instead be due to quality control, which in this case could favor stability. In transgenic tobacco, a mutated phaseolin form, that is unable to assemble and is much more susceptible to in vitro proteolysis than wild-type phaseolin, is subjected to prolonged retention in the ER by quality control and accumulates to levels comparable to the wild type counterpart (Pedrazzini et al., 1997).
It is also possible that part of the SIgA/G fragments present in the vacuole retain their activity, although most probably complete degradation of Ig occurs therein to some extent. In this respect, solving the problem that leads to partial vacuolar delivery of SIgA will not only increase our knowledge on the recognition events in the plant secretory pathway but also have practical implications for the efficient production of active SIgA.
MATERIALS AND METHODS
In Vivo Labeling of Protoplasts and Analysis of Igs
The generation of transgenic tobacco (Nicotiana tabacum) plants expressing the monoclonal Guy's 13 IgG and the derivative, hybrid SIgA/G antibody has been described (Ma et al., 1994, 1995). Protoplasts were purified from leaves of transgenic plants expressing Guy's 13 IgG, IgA/G, dIgA/G, or SIgA/G as described (Pedrazzini et al., 1994). Pulse-chase labeling of protoplasts using a mixture of [35S]Met and [35S]Cys (Pro-Mix, Amersham Pharmacia Biotech, Little Chalfont, UK), cell homogenization and immunoprecipitation were performed as described previously (Pedrazzini et al., 1997). Treatment with brefeldin A was performed by pre-incubating protoplasts for 45 min in the presence of 10 μg mL−1 brefeldin A (Boehringer Mannheim, Mannheim, Germany; stock solution 2 mg mL−1 in ethanol; a corresponding amount of ethanol was added to control protoplasts) and maintaining the same concentration of the drug for the entire pulse-chase labeling. Immunoprecipitated polypeptides were resolved onto 15% reducing or 6% (w/v) non-reducing SDS-PAGE. Gels were treated with 2,5-diphenyloxazole dissolved in dimethyl sulfoxide and radioactive proteins visualized by fluorography. Vacuolar purification was performed as described (Dombrowski et al., 1994). The recovery of vacuoles was around 30% based on α-mannosidase activity; this vacuolar fraction contained much less than 1% of the total cellular amount of the ER resident chaperone BiP, strongly suggesting very low contamination by other compartments of the secretory pathway (not shown).
For the isolation of microsomes, protoplasts were pulse-labeled for 1 h and precipitated by adding 3 volumes of W5 medium (154 mm NaCl, 5 mm KCl, 125 mm CaCl2.2H2O, and 5 mm Glc). Cells were resuspended in Suc buffer (100 mm Tris [tris(hydroxymethyl)aminomethane]-HCl, pH 7.5, 10 mm KCl, 1 mm EDTA, and 12% [w/w] Suc) and lysed on ice by sonication. The homogenate was centrifuged for 5 min at 1,000g to remove debris and intact cells. The supernatant was loaded on top of a 17% (w/w) Suc pad and centrifuged for 30 min at 150,000g in a TLA100 rotor (Beckman Instruments, Fullerton, CA), 4°C. The supernatant (containing cytosolic and vacuolar proteins) was removed and the microsomal pellet was resuspended in Suc buffer, in Suc buffer containing 1 m NaCl, or in 0.1 m Na2CO3. After 30 min of incubation on ice, the microsome suspensions were reloaded on top of a 17% (w/w) Suc pad and centrifuged for 30 min at 150,000g. Supernatants and pellets were homogenated in (final) 100 mm Tris-Cl, 100 mm NaCl, 1 mm EDTA, and 1% (v/v) Triton X-100, pH 7.5, supplemented with protease inhibitor cocktail (Complete, Boehringer Mannheim). Igs were immunoprecipitated with anti-IgG antiserum and analyzed by 6% (w/v) non-reducing SDS-PAGE and fluorography.
Immunoelectron Microscopy
Leaf specimens were taken from transgenic tobacco plants expressing SIgA/G grown in standard greenhouse conditions for 2 months. For ultrastructural analysis, leaf strips were first fixed with 4% (w/v) paraformaldehyde, 1% (w/v) glutaraldehyde in 100 mm phosphate buffer, pH 7.4, containing 5% (w/v) Suc. After rinsing in the same buffer the leaf strips were fixed again in 1% (w/v) osmium tetraoxide in buffer. Leaf strips were washed and dehydrated through an ethanol series, and embedded in Spurr's resin. Ultrathin sections of 75 nm were stained with 2% (w/v) uranyl acetate and 0.1% (w/v) lead citrate. For ultra-structure immunocytochemistry, leaf strips were fixed with 4% (w/v) paraformaldehyde and 1% (w/v) glutaldehyde. After rinsing and dehydration, the leaf specimens were embedded in LR White resin as described (Robinson et al., 1994) except all the infiltration steps were done at room temperature and the resin was polymerized at 52°C. Ultra-thin sections (silver and gold in color) were collected onto formvar-coated nickel grids, dried in air, and used in double-antibody labeling. Grids were wetted in double-distilled water and etched with saturated sodium periodate for 30 min to unmask excessive fixation. After rinsing in double distilled water, the grids were blocked with 1.5% (w/v) chicken egg albumin in phosphate buffered saline (PBS: 150 mm NaCl and 10 mm potassium phosphate, pH 7.4) for 30 min. After blocking, grids were incubated with sheep anti-rabbit secretory antibody diluted in chicken egg albumin containing blocking buffer for 1 h. This first primary antibody was rinsed away by floating the grids in PBS for 15 min. After blocking in the block buffer for 15 min, the grids were incubated with a second primary antibody, affinity-purified rabbit anti-mouse Ig (IgG + IgA + IgM, heavy and light chain; Zymed, Carlton Court, CA) diluted in the blocking buffer for 1 h. The grids were floated in PBS for 15 min to wash away the second primary antibody and blocked with 5% (v/v) normal donkey serum diluted in PBS for 30 min. Grids were then incubated with gold (12 nm)-conjugated donkey anti-sheep Ig antibody (Jackson ImmunoResearch Lab, West Grove, PA) and gold (6 nm)-conjugated donkey anti-rabbit Ig antibody diluted in PBS containing 5% (v/v) normal donkey serum for 1 h. Grids were rinsed in PBS, then in double-distilled water and air-dried. Sections were post-stained in 2% (w/v) uranyl acetate. Electron micrographs were taken with an electron microscope (H-600, Hitachi, Tokyo).
Immunofluorescence Microscopy
After purification, protoplasts from either IgG or SIgA/G-expressing plants were resuspended in MaCa buffer (0.5 m mannitol, 20 mm CaCl2, and 0.1% [w/v] MES, pH 5.7) at a concentration of 5 × 105 cells mL−1. Three hundred microliters of cell suspension was spread onto poly-Lys-coated slides (Sigma, St. Louis) and cells were allowed to adhere for 30 min at room temperature. Cells were fixed for 30 min at room temperature in MaCa buffer containing 4% (w/v) paraformaldehyde. Cells were then permeabilized by washing three times with TSW buffer (10 mm Tris-HCl, pH 7.4, 0.9% [w/v] NaCl, 0.25% [w/v] gelatin, 0.02% [w/v] SDS, and 0.1% [v/v] Triton X-100) for 10 min at room temperature. Incubation with rabbit anti-IgG antiserum (Sigma, dilution 1:300) or anti-BiP antiserum (Pedrazzini et al., 1997; dilution 1:1,000) was in the same buffer for 1 h at room temperature. After three washes in TSW, cells were incubated for 1 h at room temperature with fluorescein isothiocyanate-conjugated goat anti-rabbit secondary antibody (Sigma) at a dilution of 1:200. After three final washes in TSW, cells were mounted in Mowiol (Calbiochem, San Diego) supplemented with 2.5% (w/v) DABCO (Sigma) as an antifade agent. Cells were visualized with a Bio-Rad MRC1024 confocal laser scanning microscope equipped with a 40X oil immersion objective, at 494 nm excitation and 520 nm emission. Thickness of the optical sections was 2 μm. Laser intensity and gain controls were used as indicated in the legend to Figure 6.
ACKNOWLEDGMENTS
We thank Roberto Sitia for the helpful discussions and suggestions, and for communicating results from his laboratory before publication. We also thank Heidi Holkeri for critical reading of the manuscript.
LITERATURE CITED
- Barrieu F, Chrispeels MJ. Delivery of a secreted soluble protein to the vacuole via a membrane anchor. Plant Physiol. 1999;120:961–968. doi: 10.1104/pp.120.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek SY, Wilkins TA, Dombrowski JA, Raikhel NV. A carboxyl-terminal propeptide is necessary for proper sorting of barley lectin to vacuoles of tobacco. Plant Cell. 1990;2:1145–1155. doi: 10.1105/tpc.2.12.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boevink P, Oparka K, Santa Cruz S, Martin B, Batteridge A, Hawes C. Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network. Plant J. 1998;15:441–447. doi: 10.1046/j.1365-313x.1998.00208.x. [DOI] [PubMed] [Google Scholar]
- Brodsky JL, McCracken AA. ER protein quality control and proteasome-mediated protein degradation. Cell Dev Biol. 1999;10:507–513. doi: 10.1006/scdb.1999.0321. [DOI] [PubMed] [Google Scholar]
- Chillaron J, Haas IG. Dissociation from BiP and retrotranslocation of unassembled immunoglobulin light chains are tightly coupled to proteasome activity. Mol Biol Cell. 2000;11:217–226. doi: 10.1091/mbc.11.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CE, Herman EM, Takasaki T, Larkins BA. The maize γ-zein sequesters α-zein and stabilizes its accumulation in protein bodies of transgenic tobacco endosperm. Plant Cell. 1996;8:2335–2345. doi: 10.1105/tpc.8.12.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad U, Fiedler U. Compartment-specific accumulation of recombinant immunoglobulins in plant cells: an essential tool for antibody production and immunomodulation of physiological functions and pathogen activity. Plant Mol Biol. 1998;38:101–109. [PubMed] [Google Scholar]
- Crofts AJ, Leborgne-Castel N, Hillmer S, Robinson DG, Phillipson B, Carlsson LE, Ashford DA, Denecke J. Saturation of the endoplasmic reticulum retention machinery reveals anterograde bulk flow. Plant Cell. 1999;11:2233–2247. doi: 10.1105/tpc.11.11.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke J, Botterman J, Deblaere R. Protein secretion in plant cells can occur via a default pathway. Plant Cell. 1990;2:51–59. doi: 10.1105/tpc.2.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski JE, Gomez L, Chrispeels MJ, Raikhel NV. Targeting of proteins to the vacuole. In: Gelvin SB, Schilperoort RA, editors. Plant Molecular Biology Manual. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 1–29. [Google Scholar]
- Dombrowski JE, Schroeder MR, Bednarek SY, Raikhel NV. Determination of the functional elements within the vacuolar targeting signal of barley lectin. Plant Cell. 1993;5:587–596. doi: 10.1105/tpc.5.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio L, de Virgilio M, Prada A, Faoro F, Vitale A. Sorting of phaseolin to the vacuole is saturable and requires a short C-terminal peptide. Plant Cell. 1998;10:1031–1042. doi: 10.1105/tpc.10.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y, Hubbard AL, Fowler S, Lazarow PB. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982;93:97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez L, Chrispeels MJ. Tonoplast and soluble vacuolar proteins are targeted by different mechanisms. Plant Cell. 1993;5:1113–1124. doi: 10.1105/tpc.5.9.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenzi S, Fra AM, Sparvoli A, Bet P, Rocco M, Sitia R. The efficiency of cysteine-mediated intracellular retention determines the differential fate of secretory IgA and IgM in B and plasma cells. Eur J Immunol. 1994;24:2477–2482. doi: 10.1002/eji.1830241033. [DOI] [PubMed] [Google Scholar]
- Hiatt A, Cafferkey R, Bowdish K. Production of antibodies in transgenic plants. Nature. 1989;342:76–78. doi: 10.1038/342076a0. [DOI] [PubMed] [Google Scholar]
- Hinz G, Menze A, Hohl I, Vaux D. Isolation of prolegumin from developing pea seeds: its binding to endomembranes and assembly into prolegumin hexamers in the protein storage vacuole. J Exp Bot. 1997;48:139–149. [Google Scholar]
- Hong E, Davidson AR, Kaiser CA. A pathway for targeting soluble misfolded proteins to the yeast vacuole. J Cell Biol. 1996;135:623–633. doi: 10.1083/jcb.135.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt DC, Chrispeels MJ. The signal peptide of a vacuolar protein is necessary and sufficient for the efficient secretion of a cytosolic protein. Plant Physiol. 1991;96:18–25. doi: 10.1104/pp.96.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JK-C, Hein MB. Immunotherapeutic potential of antibodies produced in plants. Trends Biotechnol. 1995;13:522–527. doi: 10.1016/S0167-7799(00)89016-2. [DOI] [PubMed] [Google Scholar]
- Ma JK-C, Hiatt A, Hein M, Vine ND, Wang F, Stabila P, von Dolleweerd C, Mostov K, Lehner T. Generation and assembly of secretory antibodies in plants. Science. 1995;268:716–719. doi: 10.1126/science.7732380. [DOI] [PubMed] [Google Scholar]
- Ma JK-C, Hikmat BY, Wycoff K, Vine ND, Chargelegue D, Yu L, Hein MB, Lehner T. Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nat Med. 1998;4:601–606. doi: 10.1038/nm0598-601. [DOI] [PubMed] [Google Scholar]
- Ma JK-C, Lehner T, Stabila P, Fux CI, Hiatt A. Assembly of monoclonal antibodies with IgG1 and IgA heavy chain domains in transgenic tobacco plants. Eur J Immunol. 1994;24:131–138. doi: 10.1002/eji.1830240120. [DOI] [PubMed] [Google Scholar]
- Mancini R, Fagioli C, Fra AM, Maggioni C, Sitia R. Degradation of unassembled soluble Ig subunits by cytosolic proteasomes: evidence that retrotranslocation and degradation are coupled events. FASEB J. 2000;14:769–778. doi: 10.1096/fasebj.14.5.769. [DOI] [PubMed] [Google Scholar]
- Marty F. Plant vacuoles. Plant Cell. 1999;11:587–599. doi: 10.1105/tpc.11.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K, Bassham DC, Raikhel NV, Nakamura K. Different sensitivity to wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. J Cell Biol. 1995;130:1307–1318. doi: 10.1083/jcb.130.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K, Nakamura K. Propeptide of a precursor to a plant vacuolar protein required for vacuolar targeting. Proc Natl Acad Sci USA. 1991;88:834–838. doi: 10.1073/pnas.88.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J, McGhee JR. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Neuhaus J-M, Rogers JC. Sorting of proteins to vacuoles in plant cells. Plant Mol Biol. 1998;38:127–144. [PubMed] [Google Scholar]
- Pedrazzini E, Giovinazzo G, Bielli A, de Virgilio M, Frigerio L, Pesca M, Faoro F, Bollini R, Ceriotti A, Vitale A. Protein quality control along the route to the plant vacuole. Plant Cell. 1997;9:1869–1880. doi: 10.1105/tpc.9.10.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazzini E, Giovinazzo G, Bollini R, Ceriotti A, Vitale A. Binding of BiP to an assembly-defective protein in plant cells. Plant J. 1994;5:103–110. [Google Scholar]
- Pedrazzini E, Villa A, Borgese N. A mutant cytochrome b5 with a lengthened membrane anchor escapes from the endoplasmic reticulum and reaches the plasma membrane. Proc Natl Acad Sci USA. 1996;93:4207–4212. doi: 10.1073/pnas.93.9.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueyo JJ, Chrispeels MJ, Herman EM. Degradation of transport-competent destabilized phaseolin with a signal for retention in the endoplasmic reticulum occurs in the vacuole. Planta. 1995;196:586–596. doi: 10.1007/BF00203660. [DOI] [PubMed] [Google Scholar]
- Rayon C, Lerouge P, Faye L. The protein N-glycosylation in plants. J Exp Bot. 1998;49:1463–1472. [Google Scholar]
- Robinson DL, Kahn ML, Vance CP. Cellular localization of nodule-enhanced aspartate aminotransferase in Medicago sativa L. Planta. 1994;192:202–210. [Google Scholar]
- Saalbach G, Jung R, Kunze G, Saalbach I, Adler K, Müntz K. Different legumin protein domains act as vacuolar targeting signals. Plant Cell. 1991;3:695–708. doi: 10.1105/tpc.3.7.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot AA, Raikhel NV. The specificity of vesicle trafficking: coat proteins and SNAREs. Plant Cell. 1999;11:629–641. doi: 10.1105/tpc.11.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder MR, Borkhsenious ON, Matsuoka K, Nakamura K, Raikhel NV. Colocalization of barley lectin and sporamin in vacuoles of transgenic tobacco plants. Plant Physiol. 1993;101:451–458. doi: 10.1104/pp.101.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia R, Neuberger M, Alberini C, Bet P, Fra A, Valetti C, Williams G, Milstein C. Developmental regulation of IgM secretion: the role of the carboxy-terminal cysteine. Cell. 1990;60:781–790. doi: 10.1016/0092-8674(90)90092-s. [DOI] [PubMed] [Google Scholar]
- Tabe LM, Wardley-Richardson T, Ceriotti A, Aryan A, McNabb W, Moore A, Higgins TJV. A biotechnological approach to improving the nutritive value of alfalfa. J Anim Sci. 1995;73:2752–2759. doi: 10.2527/1995.7392752x. [DOI] [PubMed] [Google Scholar]
- Underdown BJ, Dorrington KJ. Studies on the structural and conformational basis for the relative resistance of serum and secretory immunoglobulin A to proteolysis. J Immunol. 1974;112:949–959. [PubMed] [Google Scholar]
- Vitale A, Denecke J. The endoplasmic reticulum: gateway of the secretory pathway. Plant Cell. 1999;11:615–628. doi: 10.1105/tpc.11.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale A, Raikhel NV. What do proteins need to reach different vacuoles? Trends Plant Sci. 1999;4:149–155. doi: 10.1016/s1360-1385(99)01389-8. [DOI] [PubMed] [Google Scholar]
- Wandelt CI, Khan MRI, Craig S, Schroeder HE, Spencer D, Higgins TJV. Vicilin with carboxy-terminal KDEL is retained in the endoplasmic reticulum and accumulates to high levels in the leaves of transgenic plants. Plant J. 1992;2:181–192. doi: 10.1046/j.1365-313x.1992.t01-41-00999.x. [DOI] [PubMed] [Google Scholar]