Highlights
-
•
Although von Willebrand disease (VWD) is a common inherited bleeding disease, only 1 case of hepatectomy in a patient with VWD has been described.
-
•
Perioperative hemostatic management is important especially in patients with VWD undergoing surgery because of the dysfunction of the platelet and destabilization of the blood clotting factor VIII.
-
•
The patient was successfully treated by administering factor VIII/von Willebrand factor concentrate and by measuring activated partial thromboplastin time as an index for perioperative hemostatic management.
Keywords: von Willebrand disease, Hepatectomy, Activated partial thromboplastin time
Abstract
Introduction
Although von Willebrand disease (VWD) is a common inherited bleeding disorder, very few cases of surgery in patients with VWD have been reported.
Presentation of case
A 77-year-old man was referred to our hospital for treatment of hepatocellular carcinoma (HCC) based on type C chronic hepatitis. He had also been treated for VWD in the hematology department of another hospital. Partial hepatectomy was performed with the administration of factor VIII/von Willebrand factor concentrate just before and after the operation. The perioperative course was uneventful, and the patient was discharged 12 days after surgery.
Discussion
VWD causes dysfunction of the platelet and destabilization of the blood clotting factor VIII. The patient was successfully treated with measurement of activated partial thromboplastin time (APTT) as an index for the management of hemostasis.
Conclusion
This report describes a rare case of a successful perioperative management of hepatectomy in a patient with VWD.
1. Introduction
von Willebrand disease (VWD) is a common inherited bleeding disorder that often manifests clinically with hemorrhage after invasive procedures. Thus, appropriate perioperative management of hemostasis is required to prevent complications of bleeding when performing a major operation in patients with VWD.
Few cases of surgery performed for patients with VWD have been reported. To the best of our knowledge, this is the first detailed case report of hepatectomy in a patient with VWD except for 1 short report [1]. Here, we report a case of partial hepatectomy for HCC performed in a patient with VWD managed with factor VIII/von Willebrand factor (FVIII/VWF) concentrate with accurate measurement of perioperative hemostasis. This case report has been prepared in line with the SCARE criteria [2].
2. Presentation of case
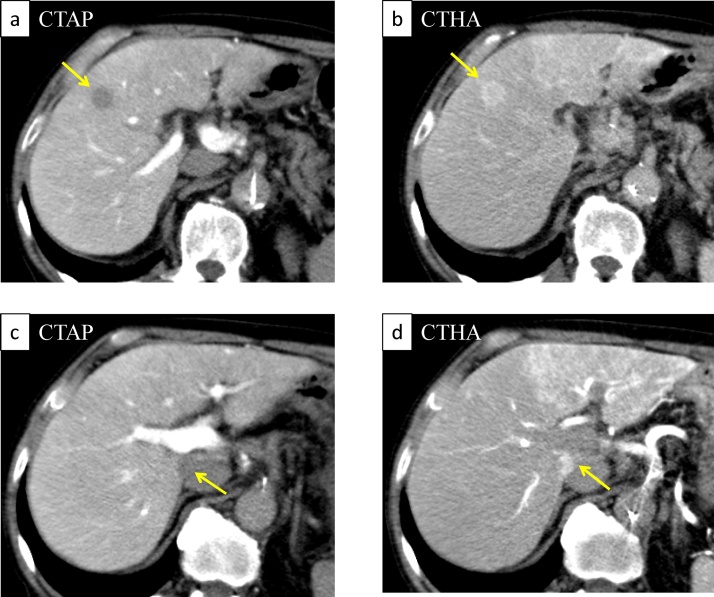
A 77-year-old man was referred to our department for surgical treatment of HCC. He had a history of recurrent nosebleeds and gastrointestinal bleeding and had been diagnosed with type 1 VWD under the care of the department of hematology in another hospital for about 70 years. He had a positive family history, and his son was also diagnosed with VWD. He also had hepatitis C, probably transmitted via the previous blood transfusion. However, he had not received therapy for hepatitis C virus yet, such as interferon therapy and direct-acting antiviral agent therapy. Contrast-enhanced computed tomography (CT) revealed a tumor in segment 5 of the liver measuring 15 mm in diameter, which was enhanced in the arterial phase and washed out in the portal and delayed phases. CT arterial portography and CT hepatic arteriography (CTAP/CTHA) revealed tumors in segment 5 and segment 1 of the liver. Both tumors were revealed defection with CTAP, and enhancement with CTHA, which were typical findings of HCC (Fig. 1). The results of the preoperative blood tests were as follows: serum aspartate aminotransferase, 56 IU/L; serum alanine aminotransferase, 41 IU/L; total serum bilirubin, 1.3 mg/dL; serum albumin, 3.7 g/dL; and prothrombin time (PT), 90%. Under the Child-Pugh classification, it was categorized as A. The serum concentration of proteins induced by vitamin K absence or antagonist was 31 mAU/mL, carbohydrate antigen 19–9 was 5.0 U/mL, and alpha-fetoprotein was 57.1 ng/mL. The indocyanine green clearance rate at 15 min was 34.2%. The serum HCV RNA was 6.5 log copies/mL.
Fig. 1.
CT arterial portography (CTAP)/CT hepatic arteriography (CTHA) image.
CTAP revealed both S5 (a) and S1 (c) tumors with perfusion defects. CTHA revealed both S5 (b) and S1 (d) tumors with enhanced image (yellow arrow).
Preoperative tests of hemostasis were as follows: the plasma VWF:ristocetin cofactor (VWF:RCo) was 6% (normal range, 60–170%) and the plasma VWF:antigen (VWF:Ag) was 24% (normal range, 50–155%); however, the plasma FVIII activity was 83% (normal range, 70–150%). Activated partial thromboplastin time (APTT) was 32.8 s and the platelet count was 12.8 × 104/mL, both within the normal range.
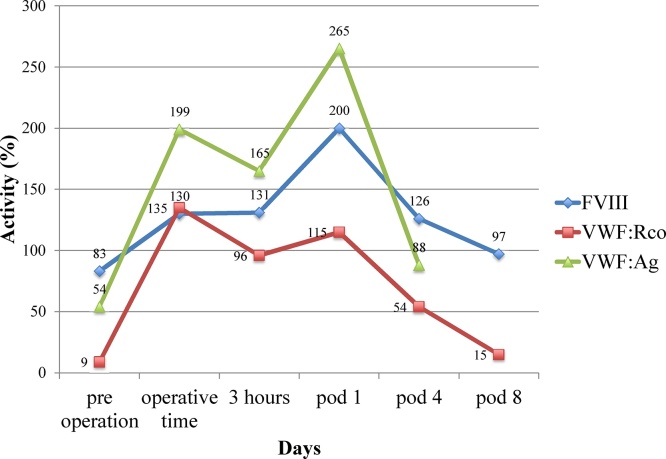
Partial hepatectomy (S5 + S1) with cholecystectomy was performed after the patient consented to undergo surgery. Although the preoperative APTT was within the normal range, we had no choice but to use the APTT as a real time index for perioperative management of hemostasis, since it took time to get the results of the plasma VWF:RCo. We administered FVIII/VWF concentrate (Confact F®; Chemo-Sero-Therapeutic Research Institute, Kumamoto, Japan), plasma-derived product, not be distributed in the world, at a dose of 3200 IU of VWF:RCo before surgery and at a dose of 1600 IU of VWF:RCo just after surgery (Fig. 2). The FVIII/VWF complex plays the key role in the intrinsic pathway of blood coagulation [3]. Therefore, we regard APTT as indexes of the activation of intrinsic coagulation and the activation of VWF. Although APTT was temporarily prolonged to 48.5 s 3 h after the start of surgery (Fig. 2), we could successfully maintain the APTT while administering the FVIII/VWF concentrate during and after the operation. Retrospectively, we could confirm that the highest activity level of FVIII increased to 200%, that of VWF:Ag to 265%, and that of VWF:RCo to 130%. The postoperative activities of FVIII, VWF:Ag, and VWF:RCo increased and peaked on the first postoperative day, and the infusion of FVIII/VWF concentrate was stopped; activity levels dropped continuously until discharge on day 12 (Fig. 3). The amount of blood loss during the operation was 484 mL. This blood loss was mainly from the parenchymal transection, although Pringle maneuver was used during the surgery (total time of Pringle maneuver: 75 min). The patient did not receive any blood transfusions during the perioperative period. The perioperative course was uneventful, and there was no complication such as bleeding. The patient was discharged from the hospital 12 days after surgery.
Fig. 2.
Serial change in APTT by supplying FVIII/VWF concentrate.
We administered VWF:Rco at a dose of 3200 IU just before surgery and 1600 IU after surgery. Although the APTT was temporarily prolonged to 48.5 s 3 h after the start of surgery, we could confirm an improvement in the APTT value. The APTT was successfully maintained at 35.9–40.2 s after surgery.
Fig. 3.
Activity of factor VIII (FVIII), von Willebrand factor antigen (VWF:Ag), and von Willebrand factor activity (VWF:RCo) during the perioperative period.
We could confirm that the highest activity level of FVIII increased to 200%, that of VWF:Ag to 265%, and that of VWF:RCo to 130%. The postoperative activity of FVIII, VWF:Ag, and VWF:RCo increased and peaked on the first postoperative day, and the infusion of FVIII/VWF concentrate was stopped; activity levels dropped continuously until discharge on day 12.
3. Discussion
VWD is a commonly inherited bleeding disorder, caused by decreased VWF activity because of either deficient or dysfunctional VWF, as first reported by Erik von Willebrand in 1926 [4]. VWF has two well-characterized functions in hemostasis: (i) the promotion of adhesion and aggregation of platelets on the sites of vascular injury and (ii) the transport and stabilization of the FVIII molecule in the plasma [5]. Thus, perioperative management of hemostasis in patients with VWD is important because of the risk of bleeding due to dysfunction of the platelet and destabilization of the blood clotting FVIII.
VWD is classified into three major categories: partial quantitative deficiency (type 1), qualitative deficiency (type 2), and total deficiency (type 3) [6]. In this case, laboratory evaluations showed a decrease in results of VWF function (VWF:RCo) and VWF protein concentration (VWF:Ag) assays; however, the blood clotting FVIII level was within the normal range. The normal or mildly decreased FVIII result, not reduced as much as that of VWF:RCo, is a characteristic finding of type 1 VWD [6]. The severity of VWD increases from type 1 to type 3 [7]. Although type 1 VWD has been described as mild [8], appropriate perioperative management is required especially for hepatectomy for HCC. This is because most HCC patients have poor hepatic functional reserve and deficient coagulation factors, and impaired synthesis of clotting factors and consumption of coagulation factors during surgery distort the coagulation balance [9].
There have been few reports of surgery in patients with VWD [[10], [11], [12]]. A MEDLINE search using the keywords “von Willebrand disease” and “hepatectomy” or “liver resection” revealed only 1 short report on hepatectomy in a patient with VWD [1]. However, details of the evaluation method of hemostasis during the perioperative period and the effect on hemostasis after administering FVIII/VWF concentrate had not been described.
In our case, the FVIII/VWF concentrate which contains about 1600 units of VWF:RCo in 1000 units was administered just before surgery and after surgery. The dosage of the FVIII/VWF concentrate was determined with reference to guidelines for coagulation factor replacement therapy for hemophilia, because there is no guideline for VWD in Japan. In case of the major surgery, “required dose (unit) = body weight (kg) × target level of VWF:RCo (%) × 0.5”. We set the target peak level of VWF:RCo as 100%, and administered 3200 units of VWF:RCo before surgery. According to the guidelines on VWD in the United States, the recommended initial dose to be administered for FVIII/VWF concentrate replacement therapy in a major surgery is 40–60 IU VWF:RCo/kg, with a maintenance dose of 20–40 IU VWF:RCo/kg administered every 8–24 h [6]. In our case, 53.3 IU VWF:RCo/kg was administered before surgery, and a half dose was once administered just after surgery while measuring the actual APTT during surgery. The highest level of VWF:Ag increased to 265%, more than our target level. As one possibility, that is because VWF derived from endothelial cells, and VWF pooled in vascular endothelial cells released into the blood by the stimulation of surgery. Administration of additional FVIII/VWF concentrate was not necessary because the APTT was maintained within the normal range, and percutaneous drainage showed no evidence of bleeding. Thromboembolic events during the treatment of patients with VWD who have high thrombotic risk and are receiving FVIII/VWF concentrate replacement therapy have been described [13,14]. Furthermore, as stated in the guidelines on VWD in the United States, individual cases may need a longer or shorter duration of additional FVIII/VWF concentrate depending on the severity of VWD and bleeding tendency [6]. Therefore, it was decided not to administer additional FVIII/VWF concentrate starting from 2 days after the surgery, in this case.
4. Conclusion
This report describes a case of a patient with type 1 VWD who underwent hepatectomy for HCC. With the appropriate perioperative management of hemostasis and use of FVIII/VWF concentrate, surgery can be performed safely without postoperative bleeding complications, even in hepatectomy.
Conflicts of interest
The authors declare that they have no competing interests.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
We have a consent by the patient. Ethical approval was obtained from the ethical committee of Hiroshima University Hospital.
Consent
Written informed consent was obtained from the patient for publication of this case report and the accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
KS and SK wrote the manuscript. KS, SK, TK and HO performed the operation. KS and SK performed the research/study, analyzed the data, designed the study, and interpreted the results. All authors conceived the study, participated in its design and coordination, and helped draft the manuscript. All authors read and approved the final manuscript.
Guarantor
Shintaro Kuroda.
References
- 1.Kokudo T., Uldry E., Degrauwe S., Demartines N., Halkic N. A successful case of right hepatectomy in a patient with von Willebrand disease. Haemophilia. 2014;20(2):e191–e192. doi: 10.1111/hae.12378. [DOI] [PubMed] [Google Scholar]
- 2.Agha R.A., Fowler A.J., Saeta A., Barai I., Rajmohan S., Orgill D.P. Erratum to The SCARE guidelines: consensus-based surgical case report guidelines [Int. J. Surg. 34 (2016) 180–186] Int. J. Surg. 2016;36(Pt A):396. doi: 10.1016/j.ijsu.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Saenko E.L., Shima M., Sarafanov A.G. Role of activation of the coagulation factor VIII in interaction with vWf, phospholipid, and functioning within the factor Xase complex. Trends Cardiovasc. Med. 1999;9(7):185–192. doi: 10.1016/s1050-1738(00)00019-0. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson I.M. The history of von Willebrand disease. Haemophilia. 1999;5(Suppl. 2):7–11. doi: 10.1046/j.1365-2516.1999.0050s2007.x. [DOI] [PubMed] [Google Scholar]
- 5.Gill J.C., Ewenstein B.M., Thompson A.R., Mueller-Velten G., Schwartz B.A. Humate PSG: Successful treatment of urgent bleeding in von Willebrand disease with factor VIII/VWF concentrate (Humate-P): use of the ristocetin cofactor assay (VWF:RCo) to measure potency and to guide therapy. Haemophilia. 2003;9(6):688–695. doi: 10.1046/j.1351-8216.2003.00816.x. [DOI] [PubMed] [Google Scholar]
- 6.Nichols W.L., Hultin M.B., James A.H., Manco-Johnson M.J., Montgomery R.R., Ortel T.L. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the national heart, lung, and blood institute (NHLBI) expert panel report (USA) Haemophilia. 2008;14(2):171–232. doi: 10.1111/j.1365-2516.2007.01643.x. [DOI] [PubMed] [Google Scholar]
- 7.Cameron C.B., Kobrinsky N. Perioperative management of patients with von Willebrand's disease. Can. J. Anaesth. 1990;37(3):341–347. doi: 10.1007/BF03005587. [DOI] [PubMed] [Google Scholar]
- 8.James P.D., Lillicrap D. von Willebrand disease: clinical and laboratory lessons learned from the large von Willebrand disease studies. Am. J. Hematol. 2012;87(Suppl. 1):S4–11. doi: 10.1002/ajh.23142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuroda S., Tashiro H., Kobayashi T., Hashimoto M., Mikuriya Y., Ohdan H. Administration of antithrombin III attenuates posthepatectomy liver failure in hepatocellular carcinoma. Dig. Surg. 2015;32(3):173–180. doi: 10.1159/000379759. [DOI] [PubMed] [Google Scholar]
- 10.Gerling V., Lahpor J.R., Buhre W. Peri-operative management of an adult patient with type 2N von Willebrand's disease scheduled for coronary artery bypass graft. Anaesthesia. 2007;62(4):405–408. doi: 10.1111/j.1365-2044.2007.05001.x. [DOI] [PubMed] [Google Scholar]
- 11.Goudemand J., Negrier C., Ounnoughene N., Sultan Y. Clinical management of patients with von Willebrand's disease with a VHP vWF concentrate: the French experience. Haemophilia. 1998;4(Suppl. 3):48–52. doi: 10.1046/j.1365-2516.1998.0040s3048.x. [DOI] [PubMed] [Google Scholar]
- 12.Ben Nsir A., Boubaker A., Jemel H. Syringomyelia following surgery for a spontaneous spinal subdural hematoma in a 13-year-old girl with congenital von Willebrand disease: case report and literature review. Childs Nerv. Syst. 2016;32(4):727–731. doi: 10.1007/s00381-015-2875-3. [DOI] [PubMed] [Google Scholar]
- 13.Makris M., Colvin B., Gupta V., Shields M.L., Smith M.P. Venous thrombosis following the use of intermediate purity FVIII concentrate to treat patients with von Willebrand's disease. Thromb. Haemost. 2002;88(3):387–388. [PubMed] [Google Scholar]
- 14.Mannucci P.M., Chediak J., Hanna W., Byrnes J., Ledford M., Ewenstein B.M. Treatment of von Willebrand disease with a high-purity factor VIII/von Willebrand factor concentrate: a prospective, multicenter study. Blood. 2002;99(2):450–456. doi: 10.1182/blood.v99.2.450. [DOI] [PubMed] [Google Scholar]