Abstract
To date, various signal transducers, cytokines, growth factors, and hormones have been reported to play an important role in homeostasis of various organs. Various cells and organs are involved in the hepatic regeneration process, which proceeds as a result of the coordination of many factors. While these factors are well known to be involved in the liver regeneration after the liver injury, however, as the details of such mechanisms have not been sufficiently elucidated, the practical applicability of hepatic regeneration based on the action of these and cytokines growth factors is still unclear. In terms of the involvement of the autonomic nervous system in hepatic regeneration, cell proliferation resulting from direct signal transduction to the liver has also been reported and recent studies focusing on the inter-organ communication via neural network opened a novel aspect of this field for therapeutic applicability. Therefore, the appropriate understanding of the relationship between autonomic neural network and liver regeneration through various organs including brain, afferent nerve, efferent nerve, etc. is essential. This mini-review explains the principle of neural system involved in the inter-organ communication and its contribution on the liver regeneration upon the liver injury reviewing recent progress in this field.
Keywords: Autonomic nerve, Neural network, Liver regeneration, Hormone
Core tip: The review of the relationship between autonomic neural network and liver regeneration shows that an inter-organ communication is functioning in a coordinated manner through the autonomic nervous system as a biological mechanism for hepatic regeneration and functional maintenance when the liver is damaged. Therefore, this mini-review presents how autonomic nerve fibers affect hepatic regeneration including the results of our most recent research.
INTRODUCTION
Many studies on the mechanisms of hepatic regeneration have led to the identification of cytokines, growth factors, and signal transducers that are produced by Kupffer cells and other cell types[1-17] which may play an important role in homeostasis of the liver (Table 1). These cytokines and growth factors are thought to cause cells enlargement and proliferation, resulting in functional recovery. However, as the details of such mechanisms have not been sufficiently elucidated, the practical applicability of hepatic regeneration based on the action of cytokines and growth factors is still unclear. Various cells and organs are involved in the hepatic regeneration process, which proceeds as a result of the coordination of many factors.
Table 1.
Factors related to the liver regeneration
| Factors related to liver regeneration | Ref. |
| Tumour necrosis factor | [3] |
| Interleukin 6 | [3] |
| NF-kappa B | [4] |
| Oncostatin M | [5] |
| Signal transducers and activator of transcription 3 | [6] |
| Hepatocyte growth factor | [7-9,11,16] |
| Epidermal growth factor | [10] |
| Transforming growth factor alpha | [10,14] |
| Epidermal growth factor receptor | [12,13] |
| Platelets | [15,16] |
| Insulin-like growth factor 1 | [16] |
| Lymphotoxin beta receptor | [17] |
| Insulin | [38] |
| Glucagon | [38] |
| Epidermal growth factor | [38] |
| Serotonin | [41,43,45,47] |
| Serotonin S2 receptor | [33] |
| Beta-catenin | [2] |
| Autonomic nervous system (direct feedback) | [18,20,31,32] |
Moreover, in terms of the involvement of the autonomic nervous system in hepatic regeneration, cell proliferation resulting from direct signal transduction to the liver has also been reported[18-20].
More specifically, this refers to actions accompanying the activation of the parasympathetic nervous system, which mediates the hepatic branch of the vagus nerve. The autonomic nervous system was first described by John N Langley in 1916[21] as an important mechanism that maintains homeostasis in organisms. These nerves are distributed internally within the blood vessels, heart, lungs, gastrointestinal tract, liver[22,23], and reproductive organs and are controlled by a feedback system that is mainly situated in the brain. While the functions of the sympathetic and parasympathetic nerves comprising the autonomic nervous system have been considered as antagonistic, they are now believed to play an important organ-related role as part of a network that maintains homeostasis in organisms. This inter-organ communication is very important in various pathologies. For example, intestinal bacterial flora have been found to be strongly involved in Parkinson’s disease[24] and autism[25], and a system to control blood sugar has been discovered in which pancreatic beta cells proliferate via autonomic nervous system signaling following a hepatectomy[26,27-29].
These results suggest that an inter-organ network functions in a coordinated manner through the autonomic nervous system as a biological mechanism for hepatic regeneration and functional maintenance when the liver is damaged[30]. This mini-review describes how autonomic nerve fibers affect hepatic regeneration based on our recent research results.
IMPACT OF AUTONOMIC NERVE FIBERS DISTRIBUTED IN THE LIVER ON HEPATIC REGENERATION
Reportedly, in addition to humoral factors, the autonomic nervous system is also involved in the hepatic regeneration process. Most studies have examined direct feedback relationship between the liver and brain. Signals starting in the liver are transmitted via the afferent sympathetic nervous system to the ventromedial region of the hypothalamus and then to the lateral region of the hypothalamus; they then pass through the dorsal nucleus of the vagus nerve of the medulla oblongata after which they return to the liver[18,19]. Kiba et al[20]. reported that in rats that had undergone partial hepatectomy, hepatic regeneration was slow when the hepatic branch of the vagus nerve was resected. In addition, they demonstrated that when the ventromedial region of the hypothalamus, which is the center of the sympathetic nervous system, was destroyed, vagus nerve signal transduction became excessive, thereby promoting post-hepatectomy hepatic regeneration[31,32]. This phenomenon occurred even if hepatectomy was not performed, and chronological evaluation showed that cell proliferation in the hepatic regeneration process peaked on day 3[33].
Thus, autonomic nervous system feedback throughout the liver is involved in hepatocyte proliferation. Kiba et al[34] also demonstrated that when the ventromedial hypothalamus, the center of the efferent sympathetic nervous system, was destroyed, pancreatic beta cells and extrapancreatic secretory cells proliferated, activating the growth of epithelial cells in the gastrointestinal tract[35]. These results demonstrated the importance of the efferent vagus nerve in the activation of cell proliferation in various organs and suggested that when the liver is injured, neural signals relayed from the liver to various organs through the brain and efferent vagus nerve might contribute to homeostasis maintenance in the body.
IMPACT OF INTER-ORGAN NETWORKS MEDIATING HEPATIC REGENERATION VIA THE AUTONOMIC NERVOUS SYSTEM
Based on the suspected contribution of the neural network on hepatic regeneration after liver injury, we focused on the effect of various organs on liver regeneration after liver injury with respect to this network. While no reports on liver regeneration have focused on this neural network, several studies so far have focused on the pancreas as an organ controlled by the autonomic nervous system[26,27-29,36]. Briefly, marked proliferation of beta cells was noted in the pancreas following partial hepatectomy in response to signal transduction through the afferent sympathetic nervous system, brain, and efferent vagus nerve[27]. Additionally, similar results were obtained when gene transfer promoted the afferent signal transduction by ERK activation[26]. These results provide evidence that the autonomic nervous system is important in maintaining for blood sugar homeostasis after severe liver damage.
It appears that this autonomic nervous system first activates the afferent sympathetic nerve in the damaged liver, which transduces the signal to the center of the autonomic nervous system in the brain and then to the efferent vagus nerve; this results in the activation of cell proliferation in various organs inside the abdominal cavity, such as the liver, gastrointestinal tract organs, and pancreas. However, no study has focused on the effect of this system on liver regeneration. Therefore, here, we focus on this system as an effector of liver regeneration. While various factors might be involved, GI hormones are known to play an important role in hepatic regeneration.
Fujita et al[37] administered various gastrointestinal and pancreatic hormones, including glucagon, secretin, and cholecystokinin, to mice that had undergone partial hepatectomy and investigated their effects on hepatic regeneration based on weight changes and histological findings. They found that the mice exhibited liver weight increases of approximately 50% following glucagon and insulin administration and suggested that this effect was mainly due to hypertrophy of the remaining cells. Lai et al[38] also reported that the combined administration of glucagon and insulin was effective for hepatic regeneration in rats that had undergone partial hepatectomy. These results demonstrated that gastrointestinal hormones may play an important role in hepatic regeneration.
Many gastrointestinal hormones assist in the maintenance of homeostasis in living organisms. Among these, serotonin, which is emitted by chromaffin cells in the intestines, has been known to encourage proliferation of liver cells[39,40]. Serotonin is a monoamine neurotransmitter that is synthesized by the enzyme tryptophan hydroxylase 1 in chromaffin cells[41] and is released in the intestine[26,27] when the parasympathetic nervous system is activated[42]. Platelet granules also contain serotonin, which is released when platelets come into contact with liver cells, after which it functions as a growth factor for liver cells[40,41,43] through the 5-HT2 receptors. Moreover, it has been reported that mice deficient in tryptophan hydroxylase 1 exhibit poor liver regeneration after hepatectomy[41].
In addition, Matondo et al[44] reported that although serotonin transporter depletion disturbed biological homeostasis, a small amount of serotonin in the liver was sufficient for liver regeneration. Mechanistically, DNA synthesis in primary rat hepatocytes cultures was induced by serotonin[33], but it was arrested by 5-HT2 receptor blockade at G1/S transition[15,45]. In the pathophysiological state, reduction of serotonin reuptake transporter function caused insulin resistance and hepatic steatosis independent of the food intake[46]; serotonin protected mouse liver from cholestatic injury by stabilizing the bile salt pool after bile duct ligation through adaptation of renal transporters in cholestasis[47].
Thus, while serotonin has been reported to act as a growth factor, no analyses of an intra-organ network, focusing on increased serotonin production in the small intestine through the afferent sympathetic nervous system and the efferent vagus nerve branch, or the promotion of hepatic regeneration have been reported.
These reports provide evidence that gastrointestinal hormones may function as growth factors upon hepatic injury. Therefore, recent studies are focusing on inter-organ communication between the liver and GI tract upon liver injury. We have reported that the effect of serotonin on liver regeneration following liver injury is mediated through this neural relay, which begins in the liver and passes through the brain and GI tract and then returns to the liver[48,49]. Our results demonstrated that the partial hepatectomy increases the serotonin release from the GI tract contributing to the liver regeneration which were evidenced by the proliferating cell nuclear antigen, BrdU incorporation and liver weight-to-body weight ratio[48,49] (see details in reference 48). In addition, this activation was blocked by neuronal blockade of the afferent visceral nerve by capsaicin suggesting that the activation of the afferent visceral nerve from the liver to the efferent vagal nerve through the brain is important[48]. Therefore, the neural relay significantly contributes in the liver regeneration upon the liver injury by activating the release of GI tract hormones as a part of maintenance of homeostasis. A summary of our studies and the reported effects of the autonomic nervous system are shown in Figure 1. This figure illustrate that while the traditionally studies have shown the direct feedback between the liver and brain have been reported, neural relay signal communicating various organs is also important, which starts from the damaged liver to the GI tract through the afferent visceral nervous system, brain, and the efferent vagal nervous system contributes to activate and release the serotonin from enterochromaffin cells to promote liver regeneration. As various factors are related to the liver regeneration (Table 1), it is obvious however, further studies are necessary to clarify the relationship between the factors.
Figure 1.
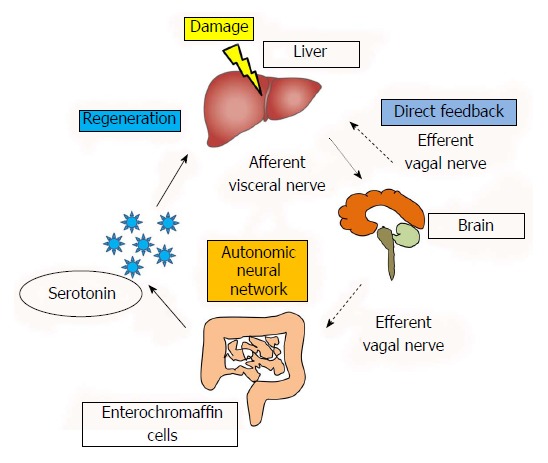
Involvement of neural signals in liver regeneration. This figure is partly reused and modified with updated information from Figure 1 in reference 49 with their permission.
CONCLUSION
The review of the relationship between autonomic neural network and liver regeneration shows that an inter-organ network is functioning in a coordinated manner through the autonomic nervous system as a biological mechanism for hepatic regeneration and functional maintenance when the liver is damaged. We believe that this study may represent a step toward the development of essential therapeutics to promote liver regeneration.
ACKNOWLEDGMENTS
The authors would like to thank Takao Tsuchida, Division of Gastroenterology and Hepatology, Niigata University for his excellent assistance in preparing the figure.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
Supported by the Brain Research Institute Grant, Niigata University.
Institutional review board statement: The study was reviewed and approved by the Institutional Review Board of Niigata University. All animal experiments were approved by and conducted in full compliance with the regulations of the Institutional Animal Care and Use Committee at Niigata University, Niigata, Japan.
Conflict-of-interest statement: The authors declare that they have no current financial arrangement or affiliation with any organization that may have a direct influence on their work.
Peer-review started: March 14, 2018
First decision: March 30, 2018
Article in press: April 16, 2018
P- Reviewer: Boscá L, Ilangumaran S, Nagaya M S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
Contributor Information
Kenya Kamimura, Division of Gastroenterology and Hepatology, Graduate School of Medical and Dental Sciences, Niigata University, Niigata 951-8510, Japan. kenya-k@med.niigata-u.ac.jp.
Ryosuke Inoue, Division of Gastroenterology and Hepatology, Graduate School of Medical and Dental Sciences, Niigata University, Niigata 951-8510, Japan.
Takuro Nagoya, Division of Gastroenterology and Hepatology, Graduate School of Medical and Dental Sciences, Niigata University, Niigata 951-8510, Japan.
Norihiro Sakai, Division of Gastroenterology and Hepatology, Graduate School of Medical and Dental Sciences, Niigata University, Niigata 951-8510, Japan.
Ryo Goto, Division of Gastroenterology and Hepatology, Graduate School of Medical and Dental Sciences, Niigata University, Niigata 951-8510, Japan.
Masayoshi Ko, Division of Gastroenterology and Hepatology, Graduate School of Medical and Dental Sciences, Niigata University, Niigata 951-8510, Japan.
Yusuke Niwa, Division of Gastroenterology and Hepatology, Graduate School of Medical and Dental Sciences, Niigata University, Niigata 951-8510, Japan.
Shuji Terai, Division of Gastroenterology and Hepatology, Graduate School of Medical and Dental Sciences, Niigata University, Niigata 951-8510, Japan.
References
- 1.Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131:1561–1572. doi: 10.1053/j.gastro.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 2.Sekine S, Gutiérrez PJ, Lan BY, Feng S, Hebrok M. Liver-specific loss of beta-catenin results in delayed hepatocyte proliferation after partial hepatectomy. Hepatology. 2007;45:361–368. doi: 10.1002/hep.21523. [DOI] [PubMed] [Google Scholar]
- 3.Iwai M, Cui TX, Kitamura H, Saito M, Shimazu T. Increased secretion of tumour necrosis factor and interleukin 6 from isolated, perfused liver of rats after partial hepatectomy. Cytokine. 2001;13:60–64. doi: 10.1006/cyto.2000.0797. [DOI] [PubMed] [Google Scholar]
- 4.Yang L, Magness ST, Bataller R, Rippe RA, Brenner DA. NF-kappaB activation in Kupffer cells after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2005;289:G530–G538. doi: 10.1152/ajpgi.00526.2004. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura K, Nonaka H, Saito H, Tanaka M, Miyajima A. Hepatocyte proliferation and tissue remodeling is impaired after liver injury in oncostatin M receptor knockout mice. Hepatology. 2004;39:635–644. doi: 10.1002/hep.20086. [DOI] [PubMed] [Google Scholar]
- 6.Moh A, Iwamoto Y, Chai GX, Zhang SS, Kano A, Yang DD, Zhang W, Wang J, Jacoby JJ, Gao B, et al. Role of STAT3 in liver regeneration: survival, DNA synthesis, inflammatory reaction and liver mass recovery. Lab Invest. 2007;87:1018–1028. doi: 10.1038/labinvest.3700630. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, Tashiro K, Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura T, Sakai K, Nakamura T, Matsumoto K. Hepatocyte growth factor twenty years on: Much more than a growth factor. J Gastroenterol Hepatol. 2011;26 Suppl 1:188–202. doi: 10.1111/j.1440-1746.2010.06549.x. [DOI] [PubMed] [Google Scholar]
- 9.Fujiwara K, Nagoshi S, Ohno A, Hirata K, Ohta Y, Mochida S, Tomiya T, Higashio K, Kurokawa K. Stimulation of liver growth by exogenous human hepatocyte growth factor in normal and partially hepatectomized rats. Hepatology. 1993;18:1443–1449. [PubMed] [Google Scholar]
- 10.Block GD, Locker J, Bowen WC, Petersen BE, Katyal S, Strom SC, Riley T, Howard TA, Michalopoulos GK. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J Cell Biol. 1996;132:1133–1149. doi: 10.1083/jcb.132.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patijn GA, Lieber A, Schowalter DB, Schwall R, Kay MA. Hepatocyte growth factor induces hepatocyte proliferation in vivo and allows for efficient retroviral-mediated gene transfer in mice. Hepatology. 1998;28:707–716. doi: 10.1002/hep.510280317. [DOI] [PubMed] [Google Scholar]
- 12.Alroy I, Yarden Y. The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997;410:83–86. doi: 10.1016/s0014-5793(97)00412-2. [DOI] [PubMed] [Google Scholar]
- 13.Linggi B, Carpenter G. ErbB receptors: new insights on mechanisms and biology. Trends Cell Biol. 2006;16:649–656. doi: 10.1016/j.tcb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Mead JE, Fausto N. Transforming growth factor alpha may be a physiological regulator of liver regeneration by means of an autocrine mechanism. Proc Natl Acad Sci USA. 1989;86:1558–1562. doi: 10.1073/pnas.86.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murata S, Ohkohchi N, Matsuo R, Ikeda O, Myronovych A, Hoshi R. Platelets promote liver regeneration in early period after hepatectomy in mice. World J Surg. 2007;31:808–816. doi: 10.1007/s00268-006-0772-3. [DOI] [PubMed] [Google Scholar]
- 16.Matsuo R, Ohkohchi N, Murata S, Ikeda O, Nakano Y, Watanabe M, Hisakura K, Myronovych A, Kubota T, Narimatsu H, et al. Platelets Strongly Induce Hepatocyte Proliferation with IGF-1 and HGF In Vitro. J Surg Res. 2008;145:279–286. doi: 10.1016/j.jss.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 17.Anders RA, Subudhi SK, Wang J, Pfeffer K, Fu YX. Contribution of the lymphotoxin beta receptor to liver regeneration. J Immunol. 2005;175:1295–1300. doi: 10.4049/jimmunol.175.2.1295. [DOI] [PubMed] [Google Scholar]
- 18.Kiba T. The role of the autonomic nervous system in liver regeneration and apoptosis--recent developments. Digestion. 2002;66:79–88. doi: 10.1159/000065594. [DOI] [PubMed] [Google Scholar]
- 19.Hendrickse MT, Thuluvath PJ, Triger DR. Natural history of autonomic neuropathy in chronic liver disease. Lancet. 1992;339:1462–1464. doi: 10.1016/0140-6736(92)92042-e. [DOI] [PubMed] [Google Scholar]
- 20.Kiba T, Tanaka K, Numata K, Hoshino M, Inoue S. Facilitation of liver regeneration after partial hepatectomy by ventromedial hypothalamic lesions in rats. Pflugers Arch. 1994;428:26–29. doi: 10.1007/BF00374748. [DOI] [PubMed] [Google Scholar]
- 21.Langley JN. Sketch of the progress of discovery in the eighteenth century as regards the autonomic nervous system. J Physiol. 1916;50:225–258. doi: 10.1113/jphysiol.1916.sp001751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berthoud HR. Anatomy and function of sensory hepatic nerves. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:827–835. doi: 10.1002/ar.a.20088. [DOI] [PubMed] [Google Scholar]
- 23.Sawchenko PE, Friedman MI. Sensory functions of the liver--a review. Am J Physiol. 1979;236:R5–20. doi: 10.1152/ajpregu.1979.236.1.R5. [DOI] [PubMed] [Google Scholar]
- 24.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell. 2016;167:1469–1480.e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 26.Imai J, Katagiri H, Yamada T, Ishigaki Y, Suzuki T, Kudo H, Uno K, Hasegawa Y, Gao J, Kaneko K, et al. Regulation of pancreatic beta cell mass by neuronal signals from the liver. Science. 2008;322:1250–1254. doi: 10.1126/science.1163971. [DOI] [PubMed] [Google Scholar]
- 27.Moreau F, Seyfritz E, Toti F, Sigrist S, Bietigier W, Pinget M, Kessler L. Early effects of liver regeneration on endocrine pancreas: in vivo change in islet morphology and in vitro assessment of systemic effects on β-cell function and viability in the rat model of two-thirds hepatectomy. Horm Metab Res. 2014;46:921–926. doi: 10.1055/s-0034-1389995. [DOI] [PubMed] [Google Scholar]
- 28.Araújo TG, Oliveira AG, Saad MJ. Partial-Hepatectomized (70%) Model Shows a Correlation between Hepatocyte Growth Factor Levels and Beta-Cell Mass. Front Endocrinol (Lausanne) 2015;6:20. doi: 10.3389/fendo.2015.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Araújo TG, Oliveira AG, Carvalho BM, Guadagnini D, Protzek AO, Carvalheira JB, Boschero AC, Saad MJ. Hepatocyte growth factor plays a key role in insulin resistance-associated compensatory mechanisms. Endocrinology. 2012;153:5760–5769. doi: 10.1210/en.2012-1496. [DOI] [PubMed] [Google Scholar]
- 30.Higgins GM, Anderson RM. Experimental pathology of liver. I. Restoration of liver of white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 31.Kiba T, Tanaka K, Endo O, Inoue S. Role of vagus nerve in increased DNA synthesis after hypothalamic ventromedial lesions in rat liver. Am J Physiol. 1992;262:G483–G487. doi: 10.1152/ajpgi.1992.262.3.G483. [DOI] [PubMed] [Google Scholar]
- 32.Kiba T, Tanaka K, Numata K, Saito S, Sekihara H. Hepatocyte proliferation in rats after ventromedial hypothalamic lesions: immunoreactivity patterns of proliferating cell nuclear antigen (PCNA) J Gastroenterol. 1998;33:523–528. doi: 10.1007/s005350050126. [DOI] [PubMed] [Google Scholar]
- 33.Balasubramanian S, Paulose CS. Induction of DNA synthesis in primary cultures of rat hepatocytes by serotonin: possible involvement of serotonin S2 receptor. Hepatology. 1998;27:62–66. doi: 10.1002/hep.510270111. [DOI] [PubMed] [Google Scholar]
- 34.Kiba T, Tanaka K, Numata K, Hoshino M, Misugi K, Inoue S. Ventromedial hypothalamic lesion-induced vagal hyperactivity stimulates rat pancreatic cell proliferation. Gastroenterology. 1996;110:885–893. doi: 10.1053/gast.1996.v110.pm8608899. [DOI] [PubMed] [Google Scholar]
- 35.Kiba T, Tanaka K, Endo O, Inoue S. Ventromedial hypothalamic lesions increase gastrointestinal DNA synthesis through vagus nerve in rats. Gastroenterology. 1993;104:475–484. doi: 10.1016/0016-5085(93)90416-a. [DOI] [PubMed] [Google Scholar]
- 36.Imai J, Oka Y, Katagiri H. Identification of a novel mechanism regulating β-cell mass: neuronal relay from the liver to pancreatic β-cells. Islets. 2009;1:75–77. doi: 10.4161/isl.1.1.8615. [DOI] [PubMed] [Google Scholar]
- 37.Fujita T. [Development of concept of gastro-entero-pancreatic endocrine system] Nihon Rinsho. 1974;32:668–673. [PubMed] [Google Scholar]
- 38.Lai HS, Chung YC, Chen WJ, Chen KM. Rat liver regeneration after partial hepatectomy: effects of insulin, glucagon and epidermal growth factor. J Formos Med Assoc. 1992;91:685–690. [PubMed] [Google Scholar]
- 39.Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. 2013;20:14–21. doi: 10.1097/MED.0b013e32835bc703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruddell RG, Mann DA, Ramm GA. The function of serotonin within the liver. J Hepatol. 2008;48:666–675. doi: 10.1016/j.jhep.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104–107. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- 42.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nocito A, Georgiev P, Dahm F, Jochum W, Bader M, Graf R, Clavien PA. Platelets and platelet-derived serotonin promote tissue repair after normothermic hepatic ischemia in mice. Hepatology. 2007;45:369–376. doi: 10.1002/hep.21516. [DOI] [PubMed] [Google Scholar]
- 44.Matondo RB, Punt C, Homberg J, Toussaint MJ, Kisjes R, Korporaal SJ, Akkerman JW, Cuppen E, de Bruin A. Deletion of the serotonin transporter in rats disturbs serotonin homeostasis without impairing liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2009;296:G963–G968. doi: 10.1152/ajpgi.90709.2008. [DOI] [PubMed] [Google Scholar]
- 45.Papadimas GK, Tzirogiannis KN, Panoutsopoulos GI, Demonakou MD, Skaltsas SD, Hereti RI, Papadopoulou-Daifoti Z, Mykoniatis MG. Effect of serotonin receptor 2 blockage on liver regeneration after partial hepatectomy in the rat liver. Liver Int. 2006;26:352–361. doi: 10.1111/j.1478-3231.2005.01230.x. [DOI] [PubMed] [Google Scholar]
- 46.Chen X, Margolis KJ, Gershon MD, Schwartz GJ, Sze JY. Reduced serotonin reuptake transporter (SERT) function causes insulin resistance and hepatic steatosis independent of food intake. PLoS One. 2012;7:e32511. doi: 10.1371/journal.pone.0032511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jang JH, Rickenbacher A, Humar B, Weber A, Raptis DA, Lehmann K, Stieger B, Moritz W, Soll C, Georgiev P, et al. Serotonin protects mouse liver from cholestatic injury by decreasing bile salt pool after bile duct ligation. Hepatology. 2012;56:209–218. doi: 10.1002/hep.25626. [DOI] [PubMed] [Google Scholar]
- 48.Inoue R, Kamimura K, Nagoya T, Sakai N, Yokoo T, Goto R, Ogawa K, Shinagawa-Kobayashi Y, Watanabe-Mori Y, Sakamaki A, et al. Effect of a neural relay on liver regeneration in mice: activation of serotonin release from the gastrointestinal tract. FEBS Open Bio. 2018;8:449–460. doi: 10.1002/2211-5463.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamimura K, Inoue R, Nagoya T. The effect of autonomic nervous system on liver regeneration. Kan-tan-sui (Japan) 2017;75:967–972. [Google Scholar]


