Abstract
HIV self-test kits may have the potential to increase testing rates around the globe, and thereby lead to reductions in HIV-related incidence and mortality. However, the effectiveness of these self-test kits and the issues surrounding self-testing have been greatly debated in recent years. We conducted a literature review on the acceptability, feasibility, and effectiveness of HIV self-testing (HST) around the world. Of the 28 articles abstracted, several themes of HST were explored, including behavioral risk compensation, presence of counseling, uses of HST, ability to perform the self-test, sensitivity and specificity, concordance with confirmatory testing, perceptions surrounding HST, instruction and supervision, and cost. Overall, this literature review found that this diverse group of participants generally performed HST correctly with a few exceptions, were accepting of the test if available at a relatively low cost, and preferred the oral-based HST over the blood-based test.
Keywords: HIV self-testing, acceptability, oral self-test, blood self-test, perceptions
INTRODUCTION
Since the AIDS epidemic began, there have been more than 70 million people infected globally with HIV and over 35 million deaths from AIDS (1,2). The World Health Organization (WHO), estimates that by the end of 2015 there were approximately 36.7 million people living with HIV (2). Between 2005 and 2015, there was a 45% decrease in AIDS-related deaths, due primarily to an increase in the availability of ART (3). In order to initiate ART, it is essential for people to first identify their HIV infection. Despite increases in HIV testing capacity, about 40% of people living with HIV are unaware of their status (3). It is therefore critically important to increase testing rates so that more people living with HIV are aware of their status.
HIV testing strategies have evolved over time. The first over-the-counter HIV test kits were approved by the US FDA in 1996 (4). These home collection tests required the user to collect a blood specimen that was then sent to a laboratory for testing. The home collection kit user would then be able to receive their test results over the phone, and could request additional telephone counseling or location of nearby HIV and mental health resources. In 2002, the FDA approved the first rapid HIV blood test, the OraQuick Rapid HIV-1 Antibody Test, which could be used to detect HIV-1 antibodies in fingerstick whole blood specimens (5). In 2003, the FDA approved the use of venipuncture whole blood specimens with the OraQuick Rapid HIV-1 Antibody Test (6). In 2004, OraSure, the manufacturer of the OraQuick test, was granted approval for testing on oral fluid, plasma, and HIV-2 (6). Additionally, in 2004, a Clinical Laboratory Improvement Amendments (CLIA) waiver was granted for this product allowing the product to be used in non-CLIA approved settings. Finally, in 2012, the FDA approved the OraQuick In-Home HIV Test as the first over-the-counter (OTC) rapid HIV self-test (4). The OraQuick In-Home HIV Test and other rapid HIV tests that can be used by an individual to test themselves for HIV are referred to as HIV self-testing (HST) kits. Currently, there are many types of HST kits developed for use around the world.
Since their introduction, HST kits have been subject to scrutiny by regulatory agencies, researchers, and users due to concerns associated with ethical, legal, and social issues (7). Benefits of HST include its high acceptability by individuals who fear the stigma of receiving a test at a clinic (8). Not only does testing at home ensure privacy, but it is more convenient for those who may struggle to find time to go to a clinic to receive testing. The oral HST kits have made HST more attractive to individuals who do not wish to have blood drawn and the short time period (< 30 mins) that testing and reading results takes is another attractive feature for users (9). Additionally, newer technologies have produced more accurate tests in recent years that overcome some of the limits of older tests (10,11). Limitations to HST include the lack of pre- and post-test counseling, the need for linkage to care among those testing positive, the need for confirmatory testing, and the “window period” during which any antibody test for HIV may show a negative result if the individual has been very recently infected (8,12,13). Despite these concerns, international regulatory agencies have begun approving HST kits in countries around the world. Many countries, including Kenya and the United Kingdom, have national policies governing the use of these HST kits and other countries have policies making HST illegal; however, many countries have no laws governing the use of HST kits (8). Several literature reviews have addressed HST kits, but we could not identify any that have focused specifically on aspects of HST related to self-administration and interpretation of the HST results (7,13–15). Our literature review differs from previous reviews in several ways: 1) we have limited our review to just HST, not all self-testing methods available; 2) in addition to unsupervised studies, we have included studies in which self-test users were trained, supervised, and/or coached through the process to allow for a comparison of different self-test processes in the literature; 3) we focus only on studies in which users self-administered the test and interpreted test results. Additionally, many new HST studies have been published in recent years and these studies have not been assimilated into the existing literature to provide an updated look at HST. With the increasing availability of these test kits as a tool for HIV self-diagnosis, it is imperative that there is updated scientific evidence on the limitations, strengths, acceptability, feasibility, and effectiveness of HST in various populations around the world. Thus, this literature review aims to summarize the current body of HST literature, with a focus on its accuracy, acceptability and perceptions, performance, limitations, and uses.
METHODS
For this review, we searched three databases, Scopus, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and PubMed in November, 2015. Scopus and CINAHL were searched using the following terms: HIV AND ((self test*) OR (self-test*) OR (home test*) OR (home-test*)). PubMed was searched with the following terms: (“hiv”[MeSH Terms] OR “hiv”[All Fields]) AND (home testing[All Fields] OR home test[All Fields]), and (“hiv”[MeSH Terms] OR “hiv”[All Fields]) AND (self testing[All Fields] OR self test[All Fields]). All articles were published or in press prior to November 2015. Studies needed to be published in English and involve human participants. Animal studies or studies using stored specimens were excluded from analyses as they did not involve human subjects performing self-testing. Published, full-text, observational studies and randomized trials were included. Literature review, abstracts, commentaries, and meta-analyses were excluded. Study participants in the included articles were 1) required to collect their own specimen and initiate their own HIV test, and 2) were required to interpret test results either from their own specimen or from model test results (e.g. positive, negative, and invalid) from the same type of test. Home collection kits (i.e. a system in which an individual takes a sample of his/her own saliva or blood with the kit to send to a lab, but does not conduct the HIV test themselves nor interpret the results of the test), were excluded due to not fitting our definition of HST. Two of the authors (DS and CV) screened study title and abstract, and three authors (DS, DV, and RD) independently read the remaining articles in full and approved them for inclusion. Discrepancies between authors were resolved through consensus. Authors of potentially included studies were contacted if necessary to receive confirmation of whether participants performed and interpreted the test themselves.
We identified several major themes of the studies to extract for inclusion in the table and discussion of the review. The following data from the studies were extracted for inclusion in Table 1: HIV prevalence, provision of pre-or post-test counseling, sensitivity and specificity of the test, concordance of test results, provision of confirmatory test (i.e. How was test verified?), HST kit used, type of sample (oral versus blood), whether testing was supervised or unsupervised, and type of instruction study participants received. Two authors (DS and CV) independently calculated the Effective Public Health Practice Project (EPHPP) quality assessment tool for quantitative studies and the Mixed Methods Appraisal Tool (MMAT) to assess the quality of the included studies (16,17). To assess the methodological quality of included studies, a final EPHPP quality score (weak, moderate, or strong) for quantitative studies, and a final MMAT quality score (25%, 50%, 75%, 100%) for all studies was reached through consensus. The MMAT overall quality score is the lowest score of the study components, with 100% suggesting all criteria are met for that study’s domains (quantitative, qualitative, and/or mixed methods domains). Data to be descriptively analyzed included the above aspects in addition to data on the possible limitations of HST (accuracy during the window period, risk compensation, ensuring confirmatory testing, and linkage to care), aspects related to ways in which HST may be used and/or accessed, factors impacting test performance and mistakes, perceptions and acceptability of HST by study participants, and aspects related to cost of the test.
Table 1.
Characteristics of Studies Reviewed
| First author’s last name and year published |
Study participants | Place & length of study | HIV prevalence |
Counseling provided? |
Sensitivity
& specificity &/or concordance of test results |
How was test verified? |
Type of self- test used and specimen collected |
Supervised or unsupervised? |
Type of instruction? |
Quality
of included Studies (EPHPP & MMAT) |
|---|---|---|---|---|---|---|---|---|---|---|
| Asiimwe et al. 2014 | 246 adult fisherfolk in three communities at high risk for HIV infection | Lake Edward, Uganda. Research clinics for counseling and client’s homes or private location for unsupervised HST. Approx. 2 months. | 13.4% (33/246) | Pre-and post-test counseling | Unsupervised: Sensitivity:
90% Specificity: 95.26% Supervised: Sensitivity: 100% Specificity: 99.1% |
Determine, STAT-PAK, and Unigold. Subset of samples retested with Western Blot and ELISA | OraQuick lnHome Rapid HIV-1/2 Antibody Test. Oral. | Both supervised and unsupervised testing occurred. | Ten minute, in Person demonstration on test kit use. Written instructions included in HST. | EPHPP: Strong MMAT: 100% |
| Balan et al. 2014 | 27 HIV negative (HIV-) MSM at high risk of for HIV infection. | NYC, NY, USA. Kits given to participants to administer at home. Interviews administered at research offices. 3 months. | Participants: 0%
(0/27) Partners: 5.6% (7/124) |
NR | NR | Participants: Clearview Complete HIV-1/2 Partners: NR | OraQuick Advance Rapid HIV-1/2 home test kit. Oral. | Participants: Supervised Partners: Could choose to be supervised or unsupervised. | Written instructions given to participants. Partners often tested with the participants and received demonstrations and coaching from them. | MMAT: 75% |
| Carballo-Dieguez et al. 2012 (J Sex Res) | 57 HIV negative (HIV-) MSM at high risk of for HIV infection. | NYC, NY, USA. Interviews & testing occurred at research offices. 3 months. | 0% (0/57) | NR | NR | Clearview Complete HIV-1/2 | OraQuick Advance Rapid HIV-1/2 home test kit. Oral. | Supervised | Written instructions given to participants | MMAT: 75% |
| Carballo-Dieguez et al. 2012 (AIDS Behav) | Partners of 57 HIV negative (HIV-) MSM at high risk of for HIV infection. | NYC, NY, USA. Interviews & testing occurred at research offices. 3 months. | 5.6% (7/124) | No. | NR | NR | OraQuick Advance Rapid HIV-1/2 home test kit. Oral. | Partners could choose to be supervised or unsupervised. | Partners often tested with the participants and received demonstrations and coaching from them | MMAT: 75% |
| Choko et al. 2011 | 283 adults | Urban Blantyre, Malawi. Community-health worker catchment areas. Testing done in homes. Approx. 5 months. | 18.5% (48/260) | Pre-and post-test counseling | Sensitivity 97.9% Specificity 100% Concordance: 99.2% |
Determine, Unigold, and SD Bioline HIV 1/2. | OraQuick Advance Rapid HIV-1/2 home test kit. Oral. | Supervised. | In-person demonstration prior to test. | MMAT: 100% |
| Choko et al. 2015 | 16,660 adults (≥16 years old) | Blantyre, Malawi. 14 clusters of neighborhoods. Testing done in homes. 2 years. | Self-reported Year 1:
11.8% Year 2: 6.8% |
Pre-and post-test counseling | Sensitivity 93.6% Specificity 99.9% Concordance: 99.4% |
Determine, Alere, and Uni-Gold Recombigen. | OraQuick Advance Rapid HIV-1/2 home test kit. Oral. | Unsupervised | In-person demonstration of test kit use | EPHPP: Strong MMAT: 100% |
| De La Fuente et al. 2012 | 313 participants of a street-based testing program | Madrid, Spain. Madrid Positivo, a mobile testing unit placed near areas with high-risk residents. Approx. 5 months. | 1.7% (9/519) | Pre- and post-test counseling. | Concordance: 92% | Determine HIV-1/2 Ag/Ab Combo performed by clinic staff. | Determine HIV-1/2 Ag/Ab Combo. Blood. | Supervised. | 207 participants received in-person demonstration 313 received written instructions. | EPHPP: Weak MMAT: 50% |
| Frasca et al. 2014 | 27 HIV negative (HIV-) MSM at high risk of for HIV infection. | New York City, NY, USA. Kits given to participants to administer at home. Interviews administered at research offices. 3 months. | NR | NR | NR | Participants: Clearview Complete HIV-1/2 Partners: NR | OraQuick Advance Rapid HIV-1/2 home test kit. Oral. | Partners could choose to be supervised or unsupervised. | Partners often tested with the participants and received demonstrations and coaching from them. | MMAT: 75% |
| Gaydos et al. 2011 | 478 adult ED patients | Baltimore, MD, USA. 2 urban EDs. ~21 months. | 0.8% (4/478) | NR | 99.6% concordant | Standard oral fluid test. | OraQuick Advance Rapid HIV-1/2 Antibody test. Oral. Unigold test. Blood. | Supervised. | Written instructions. | EPHPP: Moderate MMAT: 75% |
| Gaydos et al. 2013 | 467 adult ED patients | Baltimore, MD, USA. John Hopkins ED. ~7 months. | 0.2% (1/467) | NR | 100% concordant. | Standard oral fluid test. Western Blot if necessary. | OraQuick Advance Rapid HIV-1/2 Antibody Test. Oral. | Supervised. | Tablet-based instructions. Written visual aid. | EPHPP: Weak MMAT: 50% |
| Kalibaba et al. 2014 | 765 health care workers (HCWs) from 7 different hospitals. | Kenya. Hospitals for demonstration and surveys, and clients homes for the self-testing. ~3 months. | NR | NR | NR | NR | CalypteR AwareTM. Oral. | Unsupervised. | Live demonstration and video on the use of the HST (optional). Written instructions. | EPHPP: Weak MMAT: 50% |
| Kumwenda et al 2014. | 34 participants (17 heterosexual couples) | Blantyre, Malawi. Clinic for interviews, client homes for self-testing. | NR | Pre- and post-test counseling. | NR | NR | OraQuick Advance Rapid HIV-1/2 Antibody Test. Oral. | Supervised &/or semi-supervised. | Written instructions. | MMAT: 100% |
| Kurth et al. 2015 | 240 adults (≥18 years) | Eldoret, Kenya. Recruitment from a health care facility and 2 community workplace settings. <1 month. | 15% (35/240) | Pre- and post-test counseling. | Sensitivity: 89.7%b Specificity: 98.0%b |
Determine HIV-1/2, Alere, & ELISA. | OraQuick Advance Rapid HIV-1/2 Antibody Test. Oral. | Participants were unsupervised, but a subset were videotaped performing the HST steps. | Pictorial instruction sheet in English and Kiswahili with text and icons. | MMAT: 75% |
| Lee et al. 2007 | 350 adults | Singapore. 2 testing centers. ~7 months. | NR | Pre- and post-test counseling. | Sensitivity:
98.9% Specificity: 99.6% Concordance: 43.4% |
HCW tested using Abbott Determine HIV 1/2 rapid test. Blood. | Abbott Determine HIV 1/2 rapid test. Blood. | Unsupervised. | Pictorial instructions | EPHPP: Weak MMAT: 50% |
| Lippman et al., 2015 | 50 HIV-transgender women | Center for AIDS prevention and home setting, San Francisco, CA, USA. 3 month study. | 0% (0/50) | Pre-and post-test counseling | NR. | HCW tested using Clearview HIV 1/2 STAT-PAK. Blood. | OraQuick InHome HIV tests. Oral. | Unsupervised. | Demonstration, and written instructions included in the HST. | MMAT: 75% |
| MacPherson et al., 2014 | 16,660 adults. | Home and facility-based groups, Blantyre, Malawi. 6 month period. | Home group: 6%
(490/8194) Facility group: 3.3% (278/8466) |
Pre- and posttest counseling | NR | Referred to study clinics for confirmatory testing. | OraQuick Advance Rapid HIV-1/2 antibody test. Oral. | Unsupervised | In-person demonstration of test kit use. | EPHPP: Strong MMAT: 100% |
| Marley et al., 2014 | 1,137 adults (MSM, FSW, and VCT clients). | Community locations in 4 cities in Shandong Province, China. Single visit. | 11.8% (40/340) | Pre-test counseling. | Sensitivity: 77.5%b Specificity: 99.76%b Concordance: Self-read vs. HCW 93.88%b Saliva vs. blood 97%b |
Consultant retested the participants with a blood test. | Not mentioned. | Supervised. | Written instructions included in the HST. | MMAT: 50% |
| Marlin et al., 2014 | 274 adults, primarily AA MSM. 50 were interviewed. | Community-based organization, LA, CA, USA. Single visit. | 6.1% (3/49) | NR | NR | NR | OraQuick InHome HIV test. Oral. | Unsupervised | Written instructions included in the HST. | EPHPP: Weak MMAT: 50% |
| Martinez et al., 2014 | 57 MSM at high risk for HIV | Clinic and home settings, NYC, NY, USA. 3 month study. | 0% (0/57) | NR | NR | NR | OraQuick Advance Rapid HIV-1/2 home test kit. Oral. | Supervised by the potential partner. | Written instructions on how to use the kit were provided. | MMAT: 75% |
| Ng et al., 2012 | 200 at-risk adults for HIV | 4 clinics that test for HIV, Singapore. Single visit. | 20.3% (202/994) | Pre- and post-test counseling | Sensitivity: 97.4%a Specificity: 99.9%a Concordance: 99.38% |
Repeated test by a trained healthcare worker. | OraQuick ADVANCE Rapid HIV-1/2 Antibody Test. Oral. | Unsupervised. | Pictoral instruction sheet designed by the study team. | EPHPP: Weak MMAT: 75% |
| Nour et al., 2012 | 249 adult ED patients | Johns Hopkins ED, Baltimore, MD, USA. Single visit. | 1.6% (4/249) | NR | 100% concordance | Repeated oral-based test from a HCW. | OraQuick ADVANCE Rapid HIV-1/2 Antibody Test. Oral. | Unsupervised. | Given large instruction templates as a visual aid. | EPHPP: Moderate MMAT: 75% |
| Pant Pai et al., 2013 | 251 HCW | Groote Schurr Hospital, Cape Town, South Africa. Single visit. | 3.6% (9/251) | Pre- and post-test counseling | 98.7% concordant Sensitivity: 66.7% Specificity: 100% |
Confirmatory rapid and lab tests | OraQuick Rapid HIV-1/2 Antibody Test. Oral. | Unsupervised. | Video and picture instructions for self-testing. | MMAT: 50% |
| Pant Pai et al., 2014 | 145 university students | Student health clinic in a Canadian University, Montreal, Canada. Single visit. | 0% (0/145) | Pre- and post-test counseling | 100% concordance with HCW test. | ELISA blood test (done before selftesting) | OraQuick Rapid HIV-1/2 Antibody Test. Oral. | Unsupervised. | Video instructions or a pamphlet and pictoral reference guide. | MMAT: 50% |
| Peck et al., 2014 | Kenya: 150 Malawi: 47 South Africa: 54 Total: 251 adults | VCTs in Kenya, district HIV clinics in Malawi, office in community setting in South Africa. Single visit. | NR | NR | NR | NR | 5 prototype tests used. One was an oral test and 4 were fingerstick blood tests. | Supervised via a video monitor. | Pictorial and written instructions were provided with the testing kits. | MMAT: 75% |
| Schnall et al., 2015 | 21 adults aged 18-24 years old at high risk for HIV | Columbia Community Partnership for Health, USA. Single visit. | 0% (0/21) | NR | NR | NR. | Test brand not mentioned. Oral. | Supervised through a oneway mirror, but participants were aware of the supervision. | Written instructions included in the HST. | MMAT: 75% |
| Tao et al., 2014 | 220 MSM | Homes of study participants, China. | 15% (33/220) | Pre- and post-test counseling | 100% concordanceb | Western Blot used to confirm self-test positive results. | Human Immuno-deficiency Virus HIV K Antibody Rapid Test. Blood. | Unsupervised. | Online video instruction. | EPHPP: Weak MMAT: 50% |
| Volk et al., 2015 | 103 HIV- adult MSM at high risk for HIV infection. | 1 clinic in Rio de Janeiro, Brazil, and 2 clinics in Lima, Peru. 3 monthly visits. | 1.94% (2/103) | Pre- and post-test counseling | 100% concordance | Confirmed status prior to enrollment in the study. | Clearview COMPLETE HIV 1/2 Assay. Blood. | Unsupervised for the first two tests, supervised during the last follow-up visit. | Demonstration, as well as pictoral and written instructions. | EPHPP: Weak MMAT: 75% |
| Young et al., 2014 | 8 African-American and Latino MSM. | Gay and Lesbian Center, Hollywood, CA, USA. One time interview. | NR | NR | NR | NR | OraQuick ADVANCE Rapid HIV-1/2 Antibody test. Oral. | Unsupervised. | Written instructions included in the HST. | MMAT: 50% |
Not reported = NR
Analysis excludes invalid results
Analysis done on a subset of study participants
RESULTS
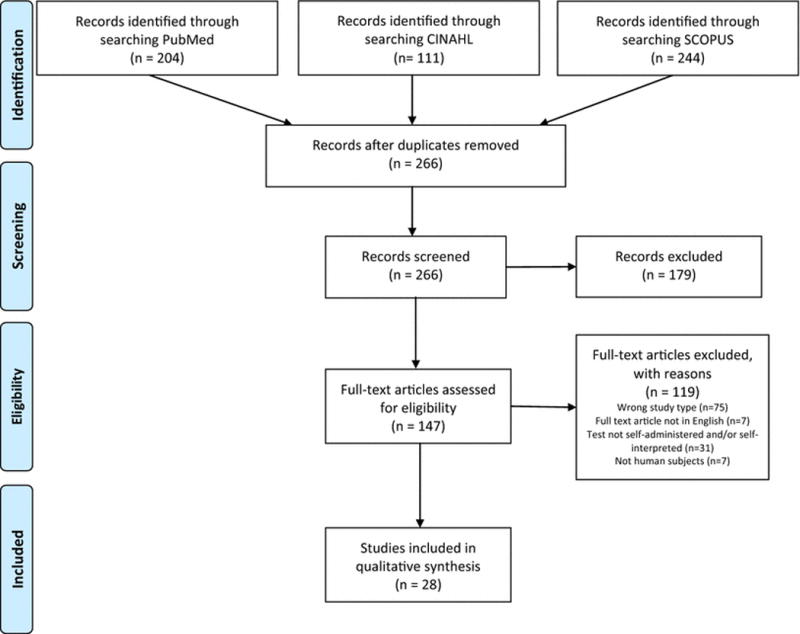
The results of our systematic search are documented in Figure 1. A total of 559 potential articles were found, with 295 duplicates, leaving 266 unique publications. We reviewed titles and abstracts for these publications and excluded those that did not meet the requirements for study design and language. The remaining 147 full text articles were assessed, and we excluded an additional 119 articles based on the full text review. No additional articles were found when manually searching references. 28 articles were included in this literature review; see characteristics of the included studies in Table I. These articles were published between 2007 and 2015, and included 21 independent studies. One study conducted on MSM in New York produced 5 articles published on qualitative and quantitative results at phases throughout the study (10,18–21). Another study was a large-scale randomized controlled trial (RCT) conducted in Malawi with nested cohort studies on HST, and had 4 published HST articles included in this review (22–25). These studies were conducted in 11 different countries, including Brazil (26), Canada (27), China (28,29), Kenya (30–32), Malawi (22–25,32), Peru (26), Singapore (33,34), Spain (11), South Africa (32,35), Uganda (36), and the United States (10,18–21,37–43). According to the World Bank country classification system, of the 11 countries, two are classified as low income countries (Uganda and Malawi), one as a lower-middle income country (Kenya), four are classified as upper-middle class countries (Brazil, China, Peru, and South Africa), and four are classified as high-income countries (Canada, Spain, Singapore, and the United States) (44). The majority of studies in this review were observational, typically crosssectional, and lacked a comparison group. However, we identified eight intervention studies or RCTs (11,18,19,22,24,25,30,36). General populations, key populations, and vulnerable populations were included in these studies. Examples of general populations were adults (23–25,31–33), heterosexual couples (22), emergency room patients (37,38,42), and university students (27). Key populations include both vulnerable and at risk populations (45). In these studies, key populations include men who have sex with men (10,18–21,26,28,29,40,43), transgender women (39), and female sex workers (28). Vulnerable populations included adults at high risk for HIV infection (34,41), fisherfolk (36), health care workers (30,35), and voluntary counseling and testing (VCT) clients (11,28). The reported HIV prevalence ranged from 0% to 20.3%, and settings for the testing included home testing (18–20,22–24,29,30,36), testing centers (11,26,32–34), specialty community centers (27,28,39–41,43), emergency rooms (37,38,42), hospitals (35), and research offices (10,21,31,32). Three of the final articles were rated as strong, two were moderate, and eight were weak according to the authors’ calculation of the EPHPP score. Using the MMAT, five articles were scored as meeting 100% of the components, thirteen articles were scored as meeting 75% of the components, and ten articles were scored as meeting 50% of the components; no included articles were scored below 50%.
Figure 1.
TEST ACCURACY
Sensitivity & specificity
Eight studies reported results regarding sensitivity and specificity of the HST kits (23,24,28,31,33–36). Six of the studies used the Oraquick Advance Rapid HIV-1/2 oral test, one used the Abbott Determine HIV 1/2 rapid blood test, and one used an unspecified HST kit. The study assessing sensitivity and specificity of the Abbott Determine rapid blood test kit reported a sensitivity of 98.9% and a specificity of 99.6%, though these values were obtained during testing performed by a trained individual (33). Sensitivity of the Oraquick Advance Rapid HIV-1/2 oral test was very low in a study conducted in a hospital in South Africa (sensitivity: 66.7%), though the training module used for the test failed to indicate that faint or weak positive lines still qualified as a positive test outcome; specificity was 100% in the same study (35). Asiimwe et al compared unsupervised and supervised HST and were unable to show non-inferiority in the sensitivity and specificity between arms (unsupervised: sensitivity 90% and specificity 95.1%; supervised: sensitivity 100% and specificity 99.1%) (36). The overall median sensitivity and specificity for the Oraquick test from all studies using this self-test kit was 93.6% and 99.9%, respectively. Marley et al found that the sensitivity of an unspecified HIV oral self-test kit was 77.5% and the specificity was 99.8% (28).
Concordance with Health Care Worker (HCW) testing or confirmatory testing
Among the articles in this literature review, 13 of the 28 articles contained evidence of confirmatory testing to corroborate the results of the self-test (11,23,24,26–29,33–35,37,38,42). The specific measures of concordance varied slightly among studies. Some studies directly compared the results acquired from the self-test and the results from the HCW, but both were read by the HCW, and some studies compared the results read from the participant and results from the HCW. Of the studies with both sets of results read by the HCW, the majority of the concordance results were quite high, including 92% (11), 97% (28), and 99.38% (23). The lowest concordance when both results were read by the HCW was a study performed by Lee et al, with a concordance of 43.4%, but this study used a blood test not designed for self-testing (33). In these aforementioned studies, the participants needed to interpret sample results, and the percentages who interpreted the sample results correctly included 95.1% (11), 88% (33), and 96%, 93.1%, and 95.2% when interpreting positive, negative, and invalid sample results, respectively (34). Of the studies comparing concordance between the results as read by the participant vs. as read by the HCW, the values were also quite high, ranging from 93.88% (28), 98.8% (35), 99.2%(23), 99.6% (37), 99.4% (24), and 100% (26,27,29,38,42).
HIV SELF-TESTING ACCEPTABILITY, PERCEPTIONS, & ATTITUDES
Acceptability
The acceptability of HST was high (range: 81%–100%), as was participants’ preference for oral HST (oral HST was preferred over an alternative testing method 81%–91% of the time) (10,24,27,28,30–32,37–39,42). Choko et al reported that, of those who chose not to use HST, participants were significantly more likely to worry about having HIV (23). Choko et al also reported that, in Malawi, the local provision of VCT by a neighbor was seen as unacceptable to many residents (64.6% of women and 38.7% of men) whereas local distribution of HST kits to neighbors without disclosing results was acceptable to 94.5% of the residents (23). Three other study conducted in Africa found that acceptability of self-testing was high due to the privacy it offered, the convenience and ease of HST, and the non-invasive nature of oral testing (22,32,35). Outside of Africa studies reported a preference for HST, though the percentage was reduced or subjects stated they had no strong preference (38,39,42). In studies where subjects had to publically retrieve their HST, the acceptability of HST was somewhat lower (40,43). Interestingly, the majority of participants opted to take their test in private and most studies reported that participants would prefer to test at home than in a clinic, despite the lack of preference for HST or VCT (33,34,38,42,43). Thus, it is likely that the convenience and privacy afforded by the home setting may be what is most preferable about HST.
Perceptions & attitudes
Most studies found that the HST kit instructions were considered to be easy to read and perform, with some variations by test type (11,26,30,33,34,37–39,41,42). Oral HST was considered to be “not at all hard” or easy to perform in the majority of studies (min: 95%, max: 99.8%) (30,34,37,38,42). A smaller percentage of participants considered blood HST to be “not at all hard” or easy to perform (min: 84%, max: 94.9%) (11,33,37). The majority of subjects felt confident that they did well reading their results. Lee et al had the largest percentage of unconfident subjects (55.5%), but this study used a kit not designed specifically for HST (33). Other authors all reported above 86% confidence in user’s ability to perform and interpret their self-test, with 100% being the highest percent of confidence reported (11,26,27,37–39,42,43).
Trust in the results of the HST was high in the majority of studies (>91%) and most participants believed their interpretations following HST were correct (>87%), with participants less likely to feel that their interpretation of a blood-based test was correct (26,27,37,42). The majority of participants would purchase their own HST kit OTC if available (min: 74%, max: 99.2%) and would recommend the self-testing process to a friend (min: 89%, max: 100%) (11,26,30,37,39).
The most common benefits of self-testing reported by participants were that the process was easy, convenient, and private. The most common limitations of self-testing reported by participants were concerns over pre- and post-test counseling, accuracy of the results, and cost of the test.
PERFORMING THE HIV SELF-TEST
Ability to perform the test
Several studies assessed performance of the test comparing the oral and blood HST. Gaydos et al found that 94% of oral and 86.7% of blood testers felt they performed the test correctly, with only one blood self-test needing to be repeated due to insufficient blood and one oral self-test being discordant with the HCW test result, but concordant with the result of a Western Blot (37). A study of five prototype HST kits found that less than half of participants collected the oral sample correctly and that performance of blood-based HST was slightly better (60.7% performed finger prick and transfer correctly) (32). However, some of the missteps performed with the blood-based testing seemed to be more severe (e.g. participants used personal items such as a razor blade to perform the finger prick) (32).
Common mistakes & factors associated with making mistakes
Overall, the most common HST mistakes included failing to prepare the test kit correctly, swabbing or taking the blood sample incorrectly, and spilling the buffer solution. Seven studies discussed common mistakes, with the percentage of subjects making mistakes ranging from 92% to only 1.1%, though the majority of studies found that few subjects made serious mistakes (e.g. drinking or spilling buffer solution and removing kit from the solution early) (11,21,26,28,31,33–36). Factors associated with studies that had a high percentage of mistakes included using a blood-based kit not designed for HST and not reading or understanding the instruction manual, especially in cases where English was not the primary language of the participants.
Multiple studies have reported variables associated with performing HST and interpreting test results. Factors that increased one’s ability to successfully perform or interpret the HST included higher education level, training prior to taking the test, younger age, prior history with HIV testing, and location of the study site in an upper-income neighborhood (11,23,33–35).
Instructions and Supervision
The studies included in this literature review varied widely in their chosen form of instruction and/or supervision of participants who self-tested, including written, pictorial, and/or video instructions, as well as demonstrations and/or supervision. Four articles stemming from the same study population described self-test kits being sent home with their participants along with written instructions on how to use the HST (10,18,20,21). The studies performed by Marlin et al and Young et al only included the written instructions already included inside the HST (40,43), and three studies only included the written instructions provided with the test kit but also with silent supervision (21,28,41). In addition to the test kit instructions, several studies provided supplementary written and/or pictorial instructions (11,22,25,33,34,38,42). As language proved to be a barrier to understanding the test kit instructions, two studies included pictorial and written instructions in both English and the local language (31,32). Additional options for instructions included internet-based and/or video instructions and/or tablet-based instruction (27,29,35,38). Seven articles mentioned a demonstration of the use of the HST prior to testing (11,23,24,26,30,36,39). In four studies, the demonstration was supplemented by written and/or illustrated instructions (23,24,26,39), and one included video instructions as well (30).
Sixteen articles involved supervision of the test (10,11,18–23,26,28,31,32,36–38,41). Three of these articles included supervision through remote means (e.g. video monitor or one-way mirror) (31,32,41). Many participants could select whether they wished their testing to be supervised or unsupervised, either by a member of the research staff, or a partner and/or friend (10,18–20). Asiimwe et al performed the only study assessing non-inferiority of unsupervised HST in comparison to the gold standard of supervised HST; in this study, they found lower sensitivity and specificity in the unsupervised HST, and therefore were unable to conclude non-inferiority (36).
HIV SELF-TESTING LIMITATIONS
The Window Period
The window period is a period of time (usually 25 days to 8 weeks) following initial infection during which the HIV antibodies may not be detectable but the viral load is high. This is a limitation of HIV testing methods that detect HIV antibodies (e.g. rapid HIV tests) as these tests are less likely to detect recent infection with HIV, resulting in a false negative test result. According to the OraQuick Advisory Committee Briefing, the comprehension of the existence of the window period was 99% among intended users of the self-test and a message concerning the window period appears on the self-test box (6). Seven of the final review articles discuss the window period as it pertains to HST (10,11,18–21,43). Three articles had participants who reported that they considered the window period to be a testing limitation (10,18,19,21). Two studies required participants to be familiar with the window period of HST (19,20).
Risk Compensation
Risk compensation is another possible limitation of HST, particularly in the case of a false-negative test result (46,47). Risk compensation refers to engaging in high-risk behavioral changes following receiving a negative HIV test result. Among our final evidence articles, four articles reported results on risk behaviors following testing, with all four reporting either a heightened awareness of risk and/or risk reduction following HST (10,18–20). However, two studies reported participants engaging in negative behavior changes following HST (e.g. not using condoms following an HIV negative test result) (18,19). On the other hand, there were a myriad of positive behavior changes linked to HST. Carballo-Dieguez et al found that “participants were 3 times more likely to report that using HT made them reduce their risk, be more cautious, practice safer sex, and to think more about whom to have sex with, than they were to report that use of HT made them more likely to have unprotected anal intercourse (UAI)” (10). In another study, upon having a partner test HIV positive using the HST kit, participants reported that no sexual contact ensued, and that emotional support and connections to health services were provided to the positive partners (20). Overall, HST seems to be more associated with positive changes in behavior, if there is any change.
Pre- and Post-test Counseling
Another limitation of HST is linkage to resources such as counseling, confirmatory testing, and treatment for HIV positive individuals (13,48,49). Among our final evidence articles, two aspects of pre- and post-test counseling emerged: the presence of pre- and post-test counseling within the study, and/or preferences mentioned by participants regarding counseling in relation to HST. Only 15 of the 28 studies (53.6%) in this literature review explicitly mention giving pre- and post-test counseling for the participants performing HST, with all 15 studies providing pre-test counseling (11,22–29,31,33–36,39), and 14 of the 15 studies providing post-test counseling (11,22–27,29,31,33–36,39). Interestingly, the majority of studies in our review reported that participants felt counseling was necessary, even for HST. Three studies reported that more than 70% of participants responded to a questionnaire on counseling by stating that pre- and post-test counseling was necessary, and Choko et al reported that alternatives to face-to-face counseling (e.g. telephone counseling and referrals to VCT) for repeat testers was not an acceptable substitute (24,33,34). In the two studies published by Pant Pai et al, participants were not asked about the necessity of counseling, but their preferred mode of counseling (27,35). The first study showed that face to face counseling was acceptable by 68.4% of participants, followed by internet/phone counseling was acceptable by 40.6% of participants (35). In the other study, 41% of participants were comfortable with either anonymous or face-to-face counseling, 39% preferred face-to-face counseling, and 16% preferred anonymous counseling (27).
Linkage to Care
Seven studies had outcomes relevant to linkage to care (20,22,24,26,29,35,40). Martinez et al found that there appeared to be a strong motivation among subjects to ensure linkage to care following a partner testing positive with an HST (20). This may suggest that linkage to care in a partner testing setting will be high, though much more research should be done on this outcome. Three studies reported 100% completion rates for linkages to care among seropositive subjects and Pant Pai et al reported 44.6% follow-up with mobile phone counseling in 242 seronegative subjects (26,35,40). A community-based prospective study conducted in Malawi reported that, across a 24 month timespan, only 56.3% of 219 HIV positive self-testers were adequately linked to care (24). However, ART initiation was used as an indicator of linkage to care and many individuals who are HIV positive may not be on ART due to local standards of care. In the same study, over 75% of subjects reported their results to a local trained counselor (24).
Cost
Ten studies reported on the cost of the HIV self-test kit (12,27,31,33,34,35,38,39,42,43). The majority of studies found that participants were not willing to pay more than 20 USD for the test, with considerable variation by country and method of test distribution. Pant Pai et al found that, in a South African population, the participants were very willing to use the oral HST kit, but would only pay between $0.10 and $6.30 for the test kit; similar results were found in a study conducted in Kenya, where participants were only willing to pay between $0.00 and $10.00 for HST (31,35). All other studies reporting on cost of the test kit were conducted in high income countries, and found that participants were willing to pay between $7 and $30 for the test kit, with a median of about $17 (11,27,33,34,38,39,42). One novel vending machine approach for dispensing HST kits conducted in the US found that the median price participants were willing to pay was $5, which is the lowest price found among high-income countries (43). Ultimately, the current cost of most HST kits exceeds what individuals are willing to pay, especially when many clinics offer free or reduced-price HIV testing using a more accurate test.
USES OF THE HIV SELF TEST
Three main uses of the test were found in our review: point-of-care testing, partner testing, and self-testing. Three studies assessed point-of-care HST where patients were asked to test themselves and read their own results prior to receiving care in an emergency department (ED) setting (37,38,42). Nine studies discussed using HST kits to screen sexual partners, a practice known as partner testing (10,18–22,30,39,41). However, five of these articles relate only to the results of a single 2-phase study conducted in a population of HIV negative gay and bisexual men (10,18–21) and two of the remaining articles only discuss the feasibility and acceptability of using an HST kit with a partner (30,41). Another article reports that, among transgender women who brought home HST kits, 3 participants used the kits to test partners, and there was overwhelming interest in partner testing using HST kits (39). Kumwenda et al reported on using HST to test as a couple and found that testing separately was viewed as limiting the lifestyle changes individuals undergo as a result of the test results and that partner testing with an HST kit increased testing rates, especially among male partners (22). Additionally, testing among couples was viewed as a way to reveal one’s HIV serostatus to one’s partner, initiate discussion concerning HIV and risk-taking behavior, and improve communication for couples (22). The final method of HST is simply self-testing and the rest of our articles fell into this category, with 16 articles involving an individual performing the process of HIV testing on themselves (11,23–29,31–36,40,43).
DISCUSSION
Twenty-eight articles involving HIV self-testing were identified for this review. We found that HST has clear potential to overcome several barriers to HIV testing by offering privacy, ease of use, and convenience. These characteristics make it a potentially useful tool for improving testing rates and access to care for seropositive individuals, especially among vulnerable and hard-to-reach populations. In addition, early evidence seems to suggest that HST has the potential to improve HIV-related communication between partners, raising awareness of one’s vulnerability to sexually transmitted infections, and as a tool to reveal one’s status to others (10,18–21). In our review, we found that the EPHPP quality metric was of limited utility for qualitative and mixed methods studies and thus included the MMAT to supplement the EPHPP scores. Overall, the evidence included in this review were of moderate quality (MMAT score of 75% and/or EPHPP scored as “moderate”); only two qualitative studies scored highly on the MMAT and three quantitative studies scored highly on both the MMAT and EPHPP quality assessments (23–26,37). Our review identified a median sensitivity and specificity of the Oraquick HIV self-test kit of 93.6% and 99.9%, respectively, and a generally high sensitivity and specificity for other test kits. As only eight studies in our review assessed the sensitivity and specificity of HST kits, with the majority of these studies focused on the Oraquick HIV self-test kit, additional research is needed in this area. However, 13 articles examined concordance of the results with a HCW and found that concordance was very high in most studies, which suggests that HST may be just as effective as clinic-based testing in countries where a rapid diagnostic test is used.
As in other reviews, we found the acceptability of HST, specifically oral HST, was high (15). This appears to be due to the convenience and privacy of testing in a home setting as opposed to a clinic, and preference for HST was strongest among areas with higher stigma against HIV, especially among African countries. This may be concerning as these populations tended to have the lowest understanding of HST packaging instructions, and thus made the most errors; additional research should be done in these populations to assess benefits and limitations to HST availability in these high-prevalence areas. In studies conducted in non-African countries, participants expressed the most interest in the convenience and ease of HST, and acceptability was slightly lower, suggesting that these populations may be more concerned with receiving the quickest and easiest HIV testing strategy. However, although HST may be an effective method for increasing HIV testing rates due to its appeal for those who wish to test privately outside the context of a clinic, the evidence suggests that it may not reach those that refuse to test due to fear of testing positive. Due to heightened acceptability of oral HST, future research should focus on oral HST as opposed to blood HST. Based on the evidence in this review, we conclude that HST is a feasible testing strategy that may have the largest impact on populations facing HIV-related stigma.
Many studies in our literature review examined HST errors, and our results suggest that most individuals were capable of performing the self-testing procedure, with discrepancies by education level and understanding of the language used in the instructions. It is likely that, in a real world scenario with an individual sufficiently motivated to purchase and use a HST kit, and improved packaging instructions available in a variety of languages and dialects, errors will be reduced.
HIV self-testing strategies varied widely in the literature. As the majority of partner testing studies using HST came from one 2-phase study conducted in New York, additional research is needed on whether partner testing may reduce sexually-transmitted HIV infection. Studies should take into account drawbacks to this strategy including partner-based violence, risk compensation, and linkage to care. There is some evidence that HST may be useful as a point-of-care testing strategy, as it would free up time for the HCW and can be performed prior to the appointment in the waiting room, though additional evidence is needed on the effectiveness of this strategy. Additional research is needed in other populations, including among heterosexual couples.
A 2014 review of the harms of self-testing concluded that there was little evidence of harms resulting from HST or other self-tests in the literature (7). Our results are largely consistent with this review, and we conclude that there appears to be a lack of evidence to the negative effect of HST limitations including the window period, risk compensation, access to appropriate pre- and post-test counseling, and ability to perform the test. Technologic advances in HIV self-testing, such as Alere Determine™ HIV-1/2 Ag/Ab Combo, which is capable of detecting the p24 antigen produced 7-9 days earlier than HIV antibodies, will allow for earlier detection of HIV and shorten the window period (10,11). Additionally, the window period and risk compensation are concerns for most HIV prevention methods, including circumcision, PREP and PEP, microbicides, and HIV vaccines, and thus are not a drawback limited to HIV self-testing. Despite concerns stated by study participants regarding self-testers receiving the necessary pre- and post-test HIV counseling, this is not a prominent drawback to the HIV self-testing strategy. In 2015, the WHO released new testing guidelines recommending against individualized counseling during pre-test information sessions and stating that a lengthy post-test counseling session for HIV negative persons has not been demonstrated to be beneficial and may detract from other HIV prevention and treatment services (50). In cases of an HIV positive diagnosis, the WHO testing guidelines recommend post-test counseling that is client-centered (50). Our results suggest that linkage to care following HST was possible, especially among self-testers with an HIV positive result, but additional research should be done assessing linkage to care, including post-test counseling, of HST in a non-clinical setting.
LIMITATIONS
Our literature review has several limitations. First, we may have missed relevant studies due to the search engines used or criteria used to assess study inclusion. Additionally, publication bias may have occurred as only published articles were included in this review. Generalizability of findings from some studies may be limited by the fact that participants had an interest in performing HST, thus elevating acceptance of the test. Few studies had a large sample size, contained a comparison group, presented longitudinal results, or examined various HST methods against each other (i.e. blood versus oral HST). Nonetheless, the studies reviewed displayed varied populations, uses of the test, and were not limited to positive findings.
CONCLUSIONS
This review highlights the benefits of HST in populations around the world, but suggests the need for larger-scale randomized trials to assess the effectiveness, acceptability, and feasibility of HST compared to clinic-based testing. With the introduction of HST to HIV-endemic countries, where acceptability for – and interest in – HST has been demonstrated to be high, it is imperative to gather an evidence base to support a decision to include or exclude HIV self-testing in country-specific HIV testing programs. This literature review examines the current body of HST literature, with a focus on the limitations and strengths, uses, perceptions and acceptability, effectiveness, and cost of HST, to provide an evidence base to inform HST-related decision-making.
Acknowledgments
The authors would like to thank Teri Lynn Herbert for her assistance in performing the literature search for this review.
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
We declare no conflicts of interest. This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.HIV/AIDS 101: Global Statistics [Internet] US Department of Health and Human Services; 2017. [cited 2017 Jan 23]. Available from: https://www.aids.gov/hiv-aids-basics/hiv-aids-101/global-statistics/index.html. [Google Scholar]
- 2.Global Health Observatory (GHO) data [Internet] World Health Organization; 2017. [cited 2017 Jan 23]. Available from: http://www.who.int/gho/hiv/en/ [Google Scholar]
- 3.The Global HIV/AIDS Epidemic [Internet] The Henry J. Kaiser Family Foundation; 2017. [cited 2017 Jan 23]. Available from: http://kff.org/global-health-policy/fact-sheet/the-global-hivaids-epidemic/#footnote-UNAIDSGlobalReport. [Google Scholar]
- 4.Hurt CB, Powers KA. Self-testing for HIV and its impact on public health. Sex Transm Dis [Internet] 2014;41(1):10–2. doi: 10.1097/OLQ.0000000000000076. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24326576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein JS. Approval Letter – OraQuick Rapid HIV-1 Antibody Test [Internet] FDA; 2002. [cited 2015 Oct 8]. Available from: http://www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/PremarketApprovalsPMAs/ucm091994.htm. [Google Scholar]
- 6.Orasure. Advisory Committee Briefing Materials : Available for Public Release Advisory Committee Briefing Materials : Available for Public Release. 2012 [Google Scholar]
- 7.Brown AN, Djimeu EW, Cameron DB. A review of the evidence of harm from self-tests. AIDS Behav. 2014;18:S445–9. doi: 10.1007/s10461-014-0831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.UNAIDS. A short technical update on self-testing for HIV. 2013 [Google Scholar]
- 9.Chen MY, Bilardi JE, Lee D, Cummings R, Bush M, Fairley CK. Australian men who have sex with men prefer rapid oral HIV testing over conventional blood testing for HIV. Int J STD AIDS. 2010;21(6):428–30. doi: 10.1258/ijsa.2010.009552. [DOI] [PubMed] [Google Scholar]
- 10.Carballo-Dieguez A, Frasca T, Balan I, Ibitoye M, Dolezal C. Use of a rapid HIV home test prevents HIV exposure in a high risk sample of men who have sex with men. AIDS Behav. 2012;16(7):1753–60. doi: 10.1007/s10461-012-0274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la Fuente L, Rosales-Statkus ME, Hoyos J, Pulido J, Santos S, Bravo MJ, et al. Are participants in a street-based HIV testing program able to perform their own rapid test and interpret the results? PLoS One [Internet] 2012;7(10):1–10. doi: 10.1371/journal.pone.0046555. Available from: http://ovid.musc.edu/prevalidate.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=medl&AN=23056342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donovan BJ, Rublein JC, Leone PA, Pilcher CD. HIV infection: point-of-care testing. Ann Pharmacother. 2004;38(4):670–6. doi: 10.1345/aph.1D314. [DOI] [PubMed] [Google Scholar]
- 13.Ibitoye M, Frasca T, Giguere R, Carballo-Dieguez A. Home testing past, present and future: lessons learned and implications for HIV home tests. AIDS Behav [Internet] 2014;18(5):933–49. doi: 10.1007/s10461-013-0668-9. Available from: http://ovid.musc.edu/prevalidate.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=medl&AN=24281697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krause J, Subklew-Sehume F, Kenyon C, Colebunders R. Acceptability of HIV self-testing: a systematic literature review. BMC Public Health [Internet] BMC Public Health. 2013;13(1):735. doi: 10.1186/1471-2458-13-735. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23924387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Figueroa C, Johnson C, Verster A, Baggaley R. AIDS Behav [Internet] Springer; US: 2015. Attitudes and Acceptability on HIV Self-testing Among Key Populations: A Literature Review. Available from: http://link.springer.com/10.1007/s10461-015-1097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armijo-Olivo S, Stiles CR, Hagen NA, Biondo PD, Cummings GG. Assessment of study quality for systematic reviews: A comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: Methodological research. J Eval Clin Pract. 2012;18(1):12–8. doi: 10.1111/j.1365-2753.2010.01516.x. [DOI] [PubMed] [Google Scholar]
- 17.Pace R, Pluye P, Bartlett G, Macaulay AC, Salsberg J, Jagosh J, et al. Testing the reliability and efficiency of the pilot Mixed Methods Appraisal Tool (MMAT) for systematic mixed studies review. Int J Nurs Stud [Internet] Elsevier Ltd. 2012;49(1):47–53. doi: 10.1016/j.ijnurstu.2011.07.002. Available from: http://dx.doi.org/10.1016/j.ijnurstu.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Frasca T, Balan I, Ibitoye M, Valladares J, Dolezal C, Carballo-Diéguez A. Attitude and Behavior Changes Among Gay and Bisexual Men After Use of Rapid Home HIV Tests to Screen Sexual Partners. AIDS Behav. 2014;9(2):1–14. doi: 10.1007/s10461-013-0630-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balan IC, Carballo-Dieguez A, Frasca T, Dolezal C, Ibitoye M. The impact of rapid HIV home test use with sexual partners on subsequent sexual behavior among men who have sex with men. AIDS Behav [Internet] 2014 Feb;18(2):254–62. doi: 10.1007/s10461-013-0497-x. [cited 2015 May 12]; Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3815512&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez O, Carballo-Diéguez A, Ibitoye M, Frasca T, Brown W, Balan I. Anticipated and Actual Reactions to Receiving HIV Positive Results Through Self-Testing Among Gay and Bisexual Men. AIDS Behav. 2014:2485–95. doi: 10.1007/s10461-014-0790-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carballo-Dieguez A, Frasca T, Dolezal C, Balan I. Will gay and bisexually active men at high risk of infection use over-the-counter rapid HIV tests to screen sexual partners? J Sex Res. 2012;49(4):379–87. doi: 10.1080/00224499.2011.647117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumwenda M, Munthali A, Phiri M, Mwale D, Gutteberg T, MacPherson E, et al. Factors shaping initial decision-making to self-test amongst cohabiting couples in urban Blantyre, Malawi. AIDS Behav [Internet] 2014;18:S396–404. doi: 10.1007/s10461-014-0817-9. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4102820&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choko AT, Desmond N, Webb EL, Chavula K, Napierala-Mavedzenge S, Gaydos CA, et al. The uptake and accuracy of oral kits for HIV self-testing in high HIV prevalence setting: a cross-sectional feasibility study in Blantyre, Malawi. PLoS Med. 2011;8(10):1–11. doi: 10.1371/journal.pmed.1001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choko AT, MacPherson P, Webb EL, Willey BA, Feasy H, Sambakunsi R, et al. PLoS Med [Internet] 9. Vol. 12. Public Library of Science; 2015. Uptake, Accuracy, Safety, and Linkage into Care over Two Years of Promoting Annual Self-Testing for HIV in Blantyre, Malawi: A Community-Based Prospective Study; p. e1001873. [cited 2015 Nov 23]; Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-84943168688&partnerID=tZOtx3y1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacPherson P, Lalloo DG, Webb EL, Maheswaran H, Choko AT, Makombe SD, et al. Effect of optional home initiation of HIV care following HIV self-testing on antiretroviral therapy initiation among adults in Malawi: a randomized clinical trial. Jama [Internet] 2014;312(4):372–9. doi: 10.1001/jama.2014.6493. [cited 2015 Nov 23] Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-84904541980&partnerID=tZOtx3y1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volk JE, Lippman SA, Grinsztejn B, Lama JR, Fernandes NM, Gonzales P, et al. Acceptability and feasibility of HIV self-testing among men who have sex with men in Peru and Brazil. Int J STD AIDS [Internet] 2015 May 12; doi: 10.1177/0956462415586676. [cited 2015 Nov 23]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/25971262. [DOI] [PMC free article] [PubMed]
- 27.Pant Pai N, Bhargava M, Joseph L, Sharma J, Pillay S, Balram B, et al. Will an unsupervised self-testing strategy be feasible to operationalize in Canada? Results from a pilot study in students of a large canadian university. AIDS Res Treat. 2014;2014:1–8. doi: 10.1155/2014/747619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marley G, Kang D, Wilson EC, Huang T, Qian Y, Li X, et al. Introducing rapid oral-fluid HIV testing among high risk populations in Shandong, China: feasibility and challenges. BMC Public Health. 2014;14(422):1–8. doi: 10.1186/1471-2458-14-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tao J, Li M, Qian H-Z, Wang L-J, Zhang Z, Ding H-F, et al. Home-based HIV testing for men who have sex with men in China: a novel community-based partnership to complement government programs. PLoS One [Internet] 2014;9(7):e102812. doi: 10.1371/journal.pone.0102812. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4106852&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalibala S, Tun W, Cherutich P, Nganga A, Oweya E, Oluoch P. Factors associated with acceptability of HIV self-testing among health care workers in Kenya. AIDS Behav [Internet] 2014;18(Suppl 4):S405–14. doi: 10.1007/s10461-014-0830-z. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24974123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurth AE, Cleland CM, Chhun N, Sidle JE, Were E, Naanyu V, et al. AIDS Behav [Internet] Springer; New York LLC: 2015. Oct 5, Accuracy and Acceptability of Oral Fluid HIV Self-Testing in a General Adult Population in Kenya. [cited 2015 Nov 23]; Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-84944624295&partnerID=tZOtx3y1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peck RB, Lim JM, Van Rooyen H, Mukoma W, Chepuka L, Bansil P, et al. What Should the IDEAL HIV self-test look like? A usability study of test prototypes in unsupervised HIV self-testing in Kenya, Malawi, and South Africa. AIDS Behav. 2014;18(SUPPL 4):422–32. doi: 10.1007/s10461-014-0818-8. [DOI] [PubMed] [Google Scholar]
- 33.Lee VJ, Tan SC, Earnest A, Seong PS, Tan HH, Leo YS. User acceptability and feasibility of self-testing with HIV rapid tests. J Acquir Immune Defic Syndr [Internet] 2007;45(4):449–53. doi: 10.1097/QAI.0b013e318095a3f3. Available from: http://ovid.musc.edu/prevalidate.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=med5&AN=17554213. [DOI] [PubMed] [Google Scholar]
- 34.Ng OT, Chow AL, Lee VJ, Chen MIC, Win MK, Tan HH, et al. Accuracy and User-Acceptability of HIV Self-Testing Using an Oral Fluid-Based HIV Rapid Test. PLoS One. 2012;7(9):e45168. doi: 10.1371/journal.pone.0045168. 2012/10/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pant Pai N, Behlim T, Abrahams L, Vadnais C, Shivkumar S, Pillay S, et al. Will an unsupervised self-testing strategy for HIV work in health care workers of South Africa? A cross sectional pilot feasibility study. PLoS One. 2013;8(11):e79772. doi: 10.1371/journal.pone.0079772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asiimwe S, Oloya J, Song X, Whalen CC. Accuracy of Un-supervised Versus Provider-Supervised Self-administered HIV Testing in Uganda: A Randomized Implementation Trial. AIDS Behav [Internet] 2014;18(12):2477–84. doi: 10.1007/s10461-014-0765-4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24691923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaydos CA, Hsieh YH, Harvey L, Burah A, Won H, Jett-Goheen M, et al. Will patients “opt in” to perform their own rapid HIV test in the emergency department? Ann Emerg Med. 2011;58(1 Suppl 1):S74–8. doi: 10.1016/j.annemergmed.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaydos CA, Solis M, Hsieh YH, Jett-Goheen M, Nour S, Rothman RE. Use of tablet-based kiosks in the emergency department to guide patient HIV self-testing with a point-of-care oral fluid test. Int J STD AIDS. 2013;24(9):716–21. doi: 10.1177/0956462413487321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lippman SA, Moran L, Sevelius J, Castillo LS, Ventura A, Treves-Kagan S, et al. AIDS Behav [Internet] Springer; New York LLC: 2015. Oct 28, Acceptability and Feasibility of HIV Self-Testing Among Transgender Women in San Francisco: A Mixed Methods Pilot Study. [cited 2015 Nov 11]; Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-84945559007&partnerID=tZOtx3y1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marlin RW, Young SD, Bristow CC, Wilson G, Rodriguez J, Ortiz J, et al. Piloting an HIV self-test kit voucher program to raise serostatus awareness of high-risk African Americans, Los Angeles. BMC Public Health [Internet] 2014;14(1226):1–5. doi: 10.1186/1471-2458-14-1226. [cited 2015 Sep 22]; Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-84928790515&partnerID=tZOtx3y1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schnall R, John RM, Carballo-Dieguez A. AIDS Behav [Internet] Springer; New York LLC: 2015. Oct 30, Do High-Risk Young Adults Use the HIV Self-Test Appropriately? Observations from a Think-Aloud Study. [cited 2015 Nov 23]; Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-84945575446&partnerID=tZOtx3y1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nour S, Hsieh YH, Rothman RE, Jett-Goheen M, Langhorne O, Wu L, et al. Patients Can Accurately Perform Their Own Rapid HIV Point-of-Care Test in the Emergency Department. Point Care. 2012;11(4):176–9. doi: 10.1097/POC.0b013e3182666eb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young SD, Daniels J, Chiu CJ, Bolan RK, Flynn RP, Kwok J, et al. Acceptability of using electronic vending machines to deliver oral rapid HIV self-testing kits: a qualitative study. PLoS One. 2014;9(7):e103790. doi: 10.1371/journal.pone.0103790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.The World Bank. Country and Lending Groups [Internet] 2015 [cited 2015 Dec 2]. Available from: http://data.worldbank.org/about/country-and-lending-groups.
- 45.The World Health Organization. Consolidated Guidelines on HIV Prevention, Diagnosis, Treatment and Care for Key Populations [Internet] 2014 Available from: http://apps.who.int/iris/bitstream/10665/128048/1/9789241507431_eng.pdf?ua=1&ua=1. [PubMed]
- 46.Walensky RP, Paltiel AD. Annals of Internal Medicine Perspective Rapid HIV Testing at Home : Does It Solve a Problem or Create One ? AT. Ann Intern Med. 2006;145(6):459–462. doi: 10.7326/0003-4819-145-6-200609190-00010. [DOI] [PubMed] [Google Scholar]
- 47.Wright AA, Katz IT. Home testing for HIV. N Engl J Med. 2006;354(5):437–40. doi: 10.1056/NEJMp058302. [DOI] [PubMed] [Google Scholar]
- 48.Schnall R, Carballo-dieguez A, Larson E. Can the HIV HOme Test Promote Access to Care? Lessons Learned from the In-home Pregnancy Test. AIDS Behav. 2015 Jul;18(2012):2496–8. doi: 10.1007/s10461-014-0798-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wood BR, Ballenger C, Stekler JD. Arguments for and against HIV self-testing. 2014:117–26. doi: 10.2147/HIV.S49083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.The World Health Organization. Consolidated Guidelines on HIV Testing Services. 5Cs: Consent, Confidentiality, Counselling, Correct Results and Connection. 2015 [PubMed] [Google Scholar]


