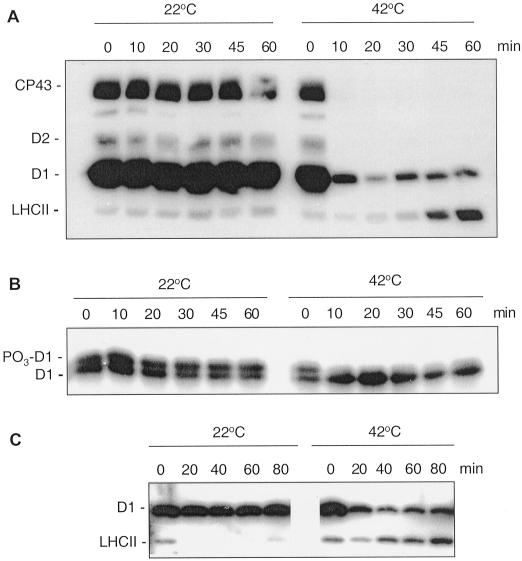
Figure 2.
Dephosphorylation of thylakoid proteins in vivo at 22°C and 42°C. Spinach leaf discs were illuminated 60 min at 22°C and then transferred to darkness and incubated at 22°C or 42°C. Dephosphorylation was terminated at the indicated time points by freezing the leaf discs in liquid nitrogen. Thylakoid membranes were isolated and the extent of protein phosphorylation was determined using a P-Thr antibody (A and C) or a D1-specific antibody (B). In the latter case the upper band of the D1 doublet represents the phosphorylated form of the protein, indicated by PO3-D1. Before conducting the dephosphorylation experiments different light intensities were used for induction of higher in vivo phosphorylation levels of either PSII core proteins or LHCII. The leaf discs were illuminated under a PFD 1,000 μmol photons m−2 s−1 for more effective phosphorylation of PSII proteins (A, B) or under a PFD 80 μmol photons m−2 s−1 for induction of LHCII phosphorylation (C).