Abstract
With growing use of nivolumab, rare but serious side effects have surfaced in some patients. We present a case of autoimmune haemolytic anaemia that developed after 39 cycles of nivolumab. A 78-year-old man with metastatic lung adenocarcinoma, refractory to multiple lines of chemotherapy was switched to nivolumab. After around 2 years of stable course on nivolumab, he developed transfusion-dependent anaemia with haemoglobin of 8.6 g/dL. Nivolumab was held immediately. Bone marrow biopsy findings were inconclusive of myelodysplastic syndrome. Further testing was suggestive of haemolysis with haptoglobin <10 mg/dL, elevated reticulocyte count and identification of immunoglobulin G antibody. Haemoglobin improved significantly with initiation of 1 mg/kg prednisone in addition to rituximab weekly × four doses. The development of transfusion-dependent anaemia with the exposure to cytotoxic chemotherapy usually raises the question for myelodysplastic syndrome. In contradiction, our patient was diagnosed to have a haematological autoimmune complication related to immunotherapy.
Keywords: haematology (drugs and medicines), malignant disease and immunosuppression, malignant and benign haematology, lung cancer (oncology)
Background
In the recent years, immunotherapy, particularly checkpoint inhibitors, has been at the forefront of cancer treatment and is increasingly being employed in multiple lines of therapy for various solid and haematological malignancies. The well-known immunotherapeutic agents are PD-1 inhibitors (nivolumab and pembrolizumab), PD-L1 inhibitors (atezolizumab and avelumab) and CTLA4 inhibitors (ipiliumumab). After revolutionising the treatment of metastatic melanoma,1 nivolumab has now been approved by Food and Drug Administration for the treatment of metastatic non-small cell lung cancer,2 metastatic renal cell cancer3 and relapsed or refractory classic Hodgkin lymphoma.4
Nivolumab, similar to other checkpoint inhibitor, can cause several immune-related adverse events, including skin rash, pneumonitis, thyroid dysfunction, hepatitis, hypophysitis, arthritis, colitis and rarely, haematological side effects.5 6 As the use of nivolumab increases, more side effects have been surfacing, among them being anaemia. Almost all cases reported were of autoimmune haemolytic anaemia (AIHA),6–10 except for a case of pure red cell aplasia (PRA) observed in the study by Yuki et al.11 Ours is a case of AIHA in a patient undergoing treatment with nivolumab for relapsed lung cancer after 39 cycles.
Case presentation
We present a case of a 78-year-old man with stage IV lung adenocarcinoma diagnosed in January 2014 with metastasis to the liver and bone. His medical history included prostate adenocarcinoma stage IIA T2a N0 M0, status post brachytherapy in 2012.
The lung cancer was initially treated with chemotherapy, starting with pemetrexed and carboplatin, followed by carboplatin, paclitaxel and bevacizumab. He then received multiple other lines of therapy including single-agent docetaxel, and finally, gemcitabine and vinorelbine. After disease progression was noted in September 2015, he was started on nivolumab 3 mg/kg, every 2 weeks.
He had finished 38 cycles of nivolumab without any complications when he developed severe anaemia after cycle 39. Initial labs drawn in April 2017 revealed haemoglobin of 8.6 g/dL (baseline 11–13 g/dL) and total bilirubin of 1.8 mg/dL. Further testing suggested haemolysis with haptoglobin <10 mg/dL and reticulocyte count elevated at 0.168 m/mcl. The patient did not report any urinary symptoms including haematuria. No improvement in anaemia was noted with vitamin B12 injections or low-dose prednisone taper starting at 40 mg/day (0.5 mg/kg/day). Nivolumab was held in the meantime, with the plan of resuming treatment once the anaemia had resolved. Repeat CT scan showed stable disease.
Investigations
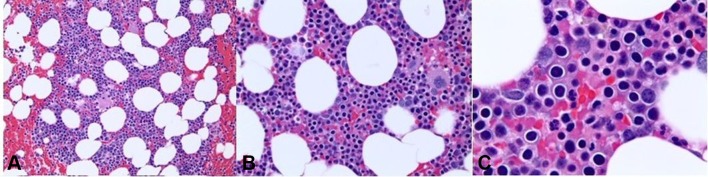
With treatment-related myelodysplastic syndrome (MDS) being a viable concern in this case, a bone marrow biopsy (figure 1) was done in June 2017, which revealed hypercellular bone marrow with erythroid hyperplasia, mild dyserythropoiesis and megaloblastoid features, with no increase in blasts, thus being inconclusive for MDS. Fluorescent in situ hybridisation and mutational profile for myelodysplastic syndrome showed negative results, and cytogenetics were normal as well. Thereafter, he was started on weekly erythropoietin (Epo) injections of 40 000 units, with only transient improvement in anaemia. He also required periodic blood transfusions due to severely low haemoglobin levels.
Figure 1.
Bone marrow biopsy demonstrating hypercellular bone marrow with erythroid hyperplasia, mild dyserythropoiesis and megaloblastoid features.
Around 4 months after he first developed anaemia, the patient was seen in our office. With positive direct Coombs test (immunoglobulin (Ig)G positive, C3 negative) and blood work consistent with haemolysis as outlined in table 1, he was diagnosed with warm autoimmune haemolytic anaemia (AIHA). Flow cytometry was done which excluded lymphoproliferative disorder and paroxysmal nocturnal haemoglobinuria. Immunofixation electrophoresis, serum protein electrophoresis and cold agglutinin resulted normal as well.
Table 1.
Anaemia workup: laboratory testing performed on referral to our office
| Workup | Result |
| Red blood cell | 2.82 m/mcl |
| Haemoglobin | 8.6 g/dL |
| Haematocrit (%) | 25.0 |
| MCV | 104 fL |
| White blood cell (×103/μL) | 10.12 k/mcl |
| Platelets (×104/μL) | 365 m/mcl |
| Reticulocytes, absolute | 0.479 m/mcl |
| Haptoglobin | <10 mg/dL |
| Indirect bilirubin (mg/dL) | 4.3 mg/dL |
| Lactate dehydrogenase (U/L) | 648 U/L |
| Direct Coombs test | (IgG +, C3 negative) |
| Bone marrow biopsy | Hypercellular bone marrow with erythroid hyperplasia, mild dyserythropoiesis and megaloblastoid features. No increase in blasts was noted |
| CT | No evidence of new neoplasms. Stable lung malignancy |
Ig, immunoglobulin; MCV, mean corpuscular volume.
Outcome and follow-up
The anaemia was considered to be caused by nivolumab therapy, as there have been other case reports describing AIHA in patients receiving PD-1 inhibitors. Since the patient had previously been treated with low-dose steroids to which he did not respond, he was started on high-dose prednisone at a dose of 1 mg/kg/day, in conjunction with weekly rituximab for four doses. Soon after initiating the high-dose steroids, he had response in haemoglobin level of 10.4 g/dL as shown in table 2. Lab test values on the day he completed rituximab showed response to treatment with haemoglobin of 11.1 g/dL, lactate dehydrogenase (LDH) of 316 U/L and total bilirubin of 1.7 mg/dL. Thereafter, the steroids have been tapered, with stable Hb, most recently being 14.6 g/dL (around 4 months since start of high-dose prednisone and rituximab). He has been off nivolumab until now with stable lung cancer on imaging and clinically, with a long-term plan of discussing re-challenge of nivolumab due to promising results in literature as shown in table 3.
Table 2.
Trend of Hb in response to trial of various treatments
| Intervention | Baseline Hb | 2 weeks after cycle 39 | 1 week after start of Vit B12 | 6 weeks after start of prednisone 40 mg/day | 3 months after Epo Intramuscular (IM) was initiated | 1 week post initiation of prednisone 1 mg/kg/day | 4 months after start of rituximab and prednisone 80 mg |
| Hb (g/dL) | 11–13 | 8.6 | 7.6 | 8.7 | 7.8 | 10.4 | 14.6 |
IM, Intramuscular.
Table 3.
Cases of anaemia observed with nivolumab in the literature
| Case report | No. of cycles before anaemia | Nivolumab dose | Type of anaemia | Primary malignancy | Mediator IgG or C3 | Treatment of AIHA | Immunotherapy re-challenge |
| Yuki et al11 | 31 cycles | Nivolumab 2 mg/kg every 6 weeks | PRA (pure red cell aplasia) | Melanoma | None | High- dose steroids | Yes, without recurrence of anaemia |
| Khan et al7 | 2 cycles | Nivolumab and ipilimumab | AIHA | Melanoma | Unknown | High-dose steroids, rituximab | Yes, with recurrence of anaemia |
| Tardy et al9 | 2 cycles | Nivolumab 3 mg/kg | AIHA | Hodgkin lymphoma | IgG | High-dose steroids | Yes, without recurrence of anaemia |
| Schwab et al10 | 8 cycles | Nivolumab 3 mg/kg every 2 weeks | AIHA | Squamous cell skin cancer | IgG, C3 | High-dose steroids | No |
| Palla et al8 | 2 cycles | Nivolumab 3 mg/kg every 2 weeks | AIHA | Metastatic lung cancer | C3 | High-dose steroids | No; patient died |
| Kong et al6 | 4 cycles | Nivolumab | AIHA | Metastatic melanoma | IgG | High-dose steroids | No |
AIHA, autoimmune haemolytic anaemia; Ig, imuunoglobulin.
Discussion
Endogenous immune checkpoints such as programmed cell death 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 terminate immune response after antigen activation,12 thereby serving as the targets for immunotherapy. PD-1 is an immunoregulatory molecule that is expressed extensively in lymphocytes and is key to immunosurveillance mechanisms. Normally PD-1 binds PD-L1 ligand on tumour cells and inactivates T cells, thus terminating immune response after antigen activation. Nivolumab blocks the binding of PD-1 and PD-L1 on tumour cells to prevent T-cell inhibition, thus amplifying the T-cell activation, which in turn targets tumour cells.12 However, this release of T-cell suppression can at times be uncontrolled and unchain a course of events that leads to an autoimmune process, thus causing the adverse effects associated with nivolumab.13
Generally, around half of AIHA cases are idiopathic.14 When a cause is known, it is usually an underlying malignancy, autoimmune disorder, drugs or infections.14 Classic laboratory findings of AIHA include normocytic or macrocytic anaemia, reticulocytosis, low serum haptoglobin levels, elevated LDH level, increased indirect bilirubin level and a positive direct antiglobulin test.
When AIHA is found in association with nivolumab, it is mostly a warm AIHA and is commonly mediated through IgG, although rare cases of C3 mediation have also been observed. In almost all cases reported, the patients received nivolumab either in the second-line or third-line setting. Usually anaemia manifests early in the treatment, after the first few cycles of nivolumab as shown in table 3. Beside our case, where patient developed anaemia after 39 cycles, the latest known in the literature is after 31 cycles in the study by Yuki et al.11 Thus, even if the patient tolerates nivolumab without haematologic complications in the first few cycles, high suspicion should be maintained for such.
It is speculated that nivolumab may be more strongly associated with AIHA compared with ipilimumab as evidenced by Kong et al6 where the patient tolerated the latter uneventfully despite the presence of prior identified red cell antibodies while developing anaemia with subsequent treatment with nivolumab.
AIHA was not observed as a significant side effect of nivolumab during the clinical trials. Anaemia was observed in 1.3% of all cases in the phase I clinical trial of nivolumab (CA209003) and in 8.6% of all cases in the phase II clinical trial (ONO-4538-02).
Due to patient’s uneventful and well-tolerated course with 38 cycles of nivolumab, reports of PD-1 inhibitors-induced AIHA usually occurring in earlier cycles and concern of treatment-related myelodysplastic syndrome, nivolumab-induced AIHA was initially low on differential of patient’s primary oncologist before he saw us. We believe that the dysplasia observed in the marrow was a result of the haemolysis, which combined with the absence of cytogenetic abnormalities or mutations for MDS makes the condition less likely. The stated reasons along with haemolysis evident on lab tests made us believe that he did not have MDS. Coombs test positive for IgG further confirmed autoimmune haemolysis as a cause of anaemia in this case. Drugs which have been generally associated with AIHA, such as beta-lactam antibiotics, cotrimoxazole, ciprofloxacin, fludarabine, lorazepam and diclofenac,14 were not relevant in our case.
The mechanism of nivolumab-induced haemolytic anaemia is not clear but is likely related to activation of autoreactive T cells and B cells, as well as suppression of T-regulatory cells.15 Recently, a few studies have demonstrated expression of PD-1 on B cells and its role in B-cell regulation and thus formation of autoantibodies.16 17 The presence of drug-induced autoantibodies is one of the most accepted aetiologies of drug-induced AIHA.
AIHA secondary to nivolumab has been usually treated with high-dose steroids,7 11 and sometimes rituximab as shown in table 3.7 Steroids work primarily by inducing immunosuppression resulting in reduction of autoantibody production. Rituximab was initially reserved for refractory and relapsed cases, however Birgens et al have demonstrated increased duration and rate of response in Warm autoimmune hemolytic anemia (WAIHA) with use of combined steroids and rituximab as first-line treatment.18 Rituximab is a chimeric monoclonal antibody with affinity for CD20-positive B lymphocytes resulting in apoptosis of the cells. Depletion of B lymphocytes leads to reduction in autoantibodies, inflammatory cytokines and T-cell activation.7 In most of the cases, the anaemia was responsive to steroids, except for Palla et al.8 Nevertheless, clinicians should have a low threshold for initiating steroids in patients with suspected nivolumab-related AIHA, and rituximab could be considered early on as an addition to steroids in first-line treatment or as a second-line agent in those who fail to respond to steroids.
Learning points.
Autoimmune haemolytic anaemia is a rare but serious side effect of nivolumab that could be refractory to treatment.
Although usually anaemia manifests after the first few cycles of nivolumab, it may still occur as a late complication (after 39 cycles).
Administering nivolumab again after such complication happens is challenging. Further studies are needed to determine the safety as well as the timing of its reuse.
Footnotes
Contributors: HS wrote the manuscript. MA contributed in the writing of case discussion along with organisation and drafting of the manuscript. ND and SF contributed to drafting, analysing and revising the manuscript critically for important intellectual content.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Redman JM, Gibney GT, Atkins MB. Advances in immunotherapy for melanoma. BMC Med 2016;14:20 10.1186/s12916-016-0571-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guibert N, Mazières J. Nivolumab for treating non-small cell lung cancer. Expert Opin Biol Ther 2015;15:1789–97. 10.1517/14712598.2015.1114097 [DOI] [PubMed] [Google Scholar]
- 3.Farolfi A, Schepisi G, Conteduca V, et al. . Pharmacokinetics, pharmacodynamics and clinical efficacy of nivolumab in the treatment of metastatic renal cell carcinoma. Expert Opin Drug Metab Toxicol 2016;12:1089–96. 10.1080/17425255.2016.1214713 [DOI] [PubMed] [Google Scholar]
- 4.Ansell SM, Lesokhin AM, Borrello I, et al. . PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 2015;372:311–9. 10.1056/NEJMoa1411087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber JS, Yang JC, Atkins MB, et al. . Toxicities of immunotherapy for the practitioner. J Clin Oncol 2015;33:2092–9. 10.1200/JCO.2014.60.0379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong BY, Micklethwaite KP, Swaminathan S, et al. . Autoimmune hemolytic anemia induced by anti-PD-1 therapy in metastatic melanoma. Melanoma Res 2016;26:202–4. 10.1097/CMR.0000000000000232 [DOI] [PubMed] [Google Scholar]
- 7.Khan U, Ali F, Khurram MS, et al. . Immunotherapy-associated autoimmune hemolytic anemia. J Immunother Cancer 2017;5:15 10.1186/s40425-017-0214-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palla AR, Kennedy D, Mosharraf H, et al. . Autoimmune hemolytic anemia as a complication of nivolumab therapy. Case Rep Oncol 2016;9:691–7. 10.1159/000452296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tardy MP, Gastaud L, Boscagli A, et al. . Autoimmune hemolytic anemia after nivolumab treatment in Hodgkin lymphoma responsive to immunosuppressive treatment. A case report. Hematol Oncol 2017;35 10.1002/hon.2338 [DOI] [PubMed] [Google Scholar]
- 10.Schwab KS, Heine A, Weimann T, et al. . Development of hemolytic anemia in a nivolumab-treated patient with refractory metastatic squamous cell skin cancer and chronic lymphatic leukemia. Case Rep Oncol 2016;9:373–8. 10.1159/000447508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuki A, Takenouchi T, Takatsuka S, et al. . A case of pure red cell aplasia during nivolumab therapy for cardiac metastatic melanoma. Melanoma Res 2017;27:635–7. 10.1097/CMR.0000000000000392 [DOI] [PubMed] [Google Scholar]
- 12.Topalian SL, Hodi FS, Brahmer JR, et al. . Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reports. PLoS One 2016;11:e0160221 10.1371/journal.pone.0160221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garbe E, Andersohn F, Bronder E, et al. . Drug induced immune haemolytic anaemia in the Berlin Case-Control Surveillance Study. Br J Haematol 2011;154:644–53. 10.1111/j.1365-2141.2011.08784.x [DOI] [PubMed] [Google Scholar]
- 15.Mqadmi A, Zheng X, Yazdanbakhsh K. CD4+CD25+ regulatory T cells control induction of autoimmune hemolytic anemia. Blood 2005;105:3746–8. 10.1182/blood-2004-12-4692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agata Y, Kawasaki A, Nishimura H, et al. . Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 1996;8:765–72. 10.1093/intimm/8.5.765 [DOI] [PubMed] [Google Scholar]
- 17.Thibult ML, Mamessier E, Gertner-Dardenne J, et al. . PD-1 is a novel regulator of human B-cell activation. Int Immunol 2013;25:129–37. 10.1093/intimm/dxs098 [DOI] [PubMed] [Google Scholar]
- 18.Birgens H, Frederiksen H, Hasselbalch HC, et al. . A phase III randomized trial comparing glucocorticoid monotherapy versus glucocorticoid and rituximab in patients with autoimmune haemolytic anaemia. Br J Haematol 2013;163:393–9. 10.1111/bjh.12541 [DOI] [PubMed] [Google Scholar]