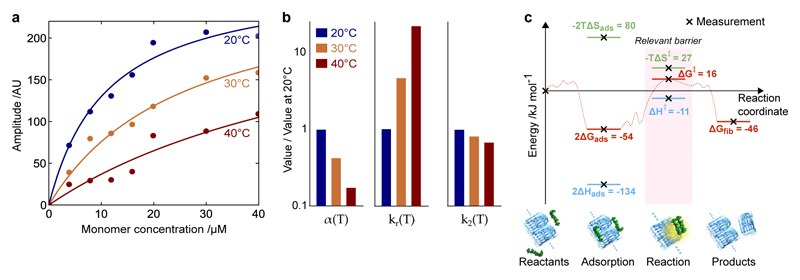
Figure 5. Mapping the energy landscape for secondary nucleation.
(a) SPR measurements of the adsorption of monomers onto fibrils as a function of concentration and temperature. The data are fitted to the Langmuir isotherm to determine KD fitted at each temperature. (b) The variation in the surface coverage α(T) = 1/KD(T), the rate constant of the subsequent nucleation reaction kr(T), and the overall rate of secondary nucleation k2(T) = α(T)2kr(T). The corresponding values of KD are KD(20°C) = 11μM, KD(30°C) = 26μM, KD(40°C) = 64μM. (c) The energy landscape for secondary nucleation assuming a standard state of 1M, showing the trajectory from starting materials, through adsorption and reaction, to products. Since our experiments are typically at micromolar concentrations, the same landscape is shown assuming a standard state of 1μM in Supplementary Fig. 6, where ΔGads > 0. Note that, according to Kramers theory of diffusive reactions, the activation parameters determined from the temperature dependence of the rate constants correspond to the highest free energy barrier measured relative to the reactants.