Abstract
Introduction
The repair of critical-sized defects (CSDs) are one of the most challenging orthopedic problems and the attempts for development of an ideal scaffold for treatment of large bone defect are ongoing.
Aim
The aim of this study was the effectiveness of hydroxyapatite-gelatin seeded with bone marrow stromal cells construct for healing of critical-sized bone defect in vivo.
Material and Methods
In this experimental study, the bone marrow stromal cells (BMSCs) were isolated by flushing method. For in vitro study, the cells were seeded on the scaffold and the cell viability as well as cytotoxicity were tested by MTT and LDH specific activity. The scaffold-cell construct was implanted into the critical-sized bone defect created in calvaria of Wistar male rats.15 rats were randomly divided into 3 groups (n=5), group 1 (control group): Injury without transplantation, group 2: implanted with hydroxyapatite-gelatin scaffold, group 3: hydroxyapatite-gelatin scaffold seeded with BMSCs. At different days post-implantation, the implanted site was collected and the bone healing was evaluated through H&E and Masson’s Trichrome staining. ANOVA and paired t-test were used for data comparison and P<0.05 was considered significant.
Results
The results of MTT showed that the scaffold has no toxic effects on stromal cells. The first signs of ossification in hydroxyapatite-gelatin with BMSCs cells group appeared in the first week. However, in the fourth week, ossification was completed and the scaffold remaining was found as embedded islands in the spongy bone tissue. The greatest number of lymphocytes in the experimental group was observed after one week of planting scaffold.
Conclusion
Hydroxyapatite-gelatin scaffold coated with BMSCs cells has a potential role in the healing process of bone and would be a possible new therapeutic strategy to repair extensive bone lesions.
Keywords: Bone marrow stromal cell, Scaffold, Tissue engineering
1. INTRODUCTION
Despite the enormous potential in the healing of the bone tissue, this process is not able to act properly under certain circumstances such as illness, trauma and compound fractures. Therefore, a recent bone defect repair by transplantation of autogenous, allogeneic, isogenic,and using synthetic materials is performed (1-3). In the reconstruction of bone defects, autologous transplant is the most preferred option. However, issues such as chronic pain, infection, nerve damage, abnormal bone formation, hemorrhage, and longer surgery duration associated with it. Allograft transplantation is another appropriate option that means transplanting an organ or tissue between two subjects of the same species genetically identical to each other. Although allograft held in tissue keeping banks is always available, risk of disease transmission and prevention of angiogenesis (vascularization) is need to be considered (4, 5). Isograft is an allograft transplantation with the difference that both the donor and recipient are genetically identical, like identical twins. This type of transplantation has the same immune response as autograft (6). Despite successful improvement in developing solutions for the defects associated with bone grafts, limitations in bone grafts and other issues, is motivating researchers for alternative materials which can be used not only in bone grafting but also in repairing bone injuries (7). One of the main issues for bone tissue engineering, is designing and constructing three-dimensional (3D) bioactive re-absorbable scaffolds that can maintain structural integrity during the recovery time. Scaffold is a provisional matrix for bone growth that provides a dedicated environment for development and to facilitate tissue adhesion, growth and differentiation of the cells. Until now, various types of scaffolds were used such as ceramic, polymers and composites. Recent studies have emphasized on applying biomaterials with bioactive materials to stimulate cell migration to the site of injury. Since bone tissue contains a mineral phase and an organic phase, to mimic scaffolds for bone matrix it is necessary to include a mineral phase (mainly hydroxyapatite) and an organic phase(proteins such as collagen or gelatin) (8). One of the composites used to prepare the gelatin is hydroxyapatite bone scaffold (9-12). Collagen is a filamentous matter, insoluble and indigestible, which becomes into gelatin by boiling in water. It was also observed that the hydroxyapatite-gelatin, is more active than collagen-hydroxyapatite in stimulating osteoblasts (13). Hydroxyapatite-Gelatin (HA-GEL) was known as an appropriate scaffold for bone formation inducing (14). Moreover, the effect of human bone matrix gelatin for the repair of bone defects in rat skull was analyzed and the results showed that bone tissue regeneration with human bone matrix gelatin were more rapidly (15). In a study in 2013, the application of hydroxyapatite-gelatin Nano composites with stem cells of uterine endometrium tissue leads to the significant formation of bone tissue (16). Since the role of bone marrow stromal cells (BMSCs) in bone tissue engineering have been widely studied and utilized as tissue repair cells. Although different types of cells have been used in tissue engineering, BMSCs cells, are appropriate for repairing injured bone tissues due to unique features such as easy access, non-stimulating antigenic properties and long-term survival and adaptation to the host tissue (17). An appropriate scaffold for bone defects need to have at least three characteristics including correct anatomical design, the ability to withstand mechanical stress and increased levels of growth factors (18).This study intends to investigate healing bone defects by changing the composite from collagen to gelatin in organic compounds and using stem cells,elevating the efficiency of repair and reducing the healing time as much as possible. Therefore in this study by conducting in vitro and transplantation on adult rat skull, we investigated the role of resulting composite in bone healing, the immune response to composite, and the effect of the scaffold on the differentiation of bone marrow mesenchymal stem cells.
2. MATERIAL AND METHODS
2.1. HA-GEL composite preparation
A 10% solution of gelatin (Merck Inc. 4070, Germany) was prepared in deionized distilled water. Then the Nano-hydroxyapatite powder (Merck Inc. 2196, Germany) was added to the solution to form a combination containing weight 60% of gel and 40% of hydroxyapatite and produced solution was put in a stirrer (Heidolph/Magnetic Stirrer & Heater) at 40°C for 45 minutes to be homogenized and followed by placing in a plastic Petri dish (to prevent the formation of bubbles or foam) to reach a thickness of 2 mm. Then the Petri dish containing the solution was quickly stored at -20° C for 1 hour until a solid layer was formed. The sample was quickly placed in a freeze dryer (EYELA/Japan) for 24 hours at the temperature of -57° C and the pressure of 0.03 Mbar until it was completely dried (9). At this stage, the product is gelatin-Nano-hydroxyapatite porous composite, that if reconstituted in water, it forms a two partial solution, and its strength will be reduced.
2.2. Extraction and culture of bone marrow-derived mesenchymal stem cells
The derivation of bone marrow mesenchymal stem cells were performed through the bones of the thigh and shin of 6-8 week aged mice (SW56), based on the Flushing method. Thus, using an insulin syringe containing DMEM/F12(10% Fetal Bovine Serum, FBS), 100units/ml penicillin (Gibco) and 100 units/ml streptomycin (Gibco) (Medium), bone marrow was extracted from both ends of the bone cuts, and all the contents of the syringe were transferred into a 25 ml cell culture flasks and cultured in an incubator at 37° C and 5% carbon dioxide and 95% humidity for a whole day. After that the medium was changed every 2 to 3 days, until all of the blood cells and hematopoietic cells were completely extracted. Bone marrow mesenchymal cells remained mostly spindle and adhered to the bottom of the flask. After filling the bottom of the flask (70-80%), cells were incubated and separated using trypsin 0.25% (Gibco). To purify mesenchymal stromal, cells were passaged 3 to 5 times.
2.3. Preparation of BMSCs-seeded HA-GEL construct
BMSCs were used at passages 3-5 times. To perform cell culture, cell counts were conducted and the number of 5-10 thousand cells were transferred to seven millimeter scaffolds that were pre-prepared and sterilized using appropriate antibiotics and placed on 6-well plates. The cells were incubated for 24 hours on the scaffolds (5% CO2, 37° C) and during this period, they were prepared for transplantation into the wound site. Alginate was used for fixing the scaffolds in the hole (in groups 2 and 3).
2.4. In vitro characterizations
The MTT test was used to study the cytotoxicity of hydroxyapatite-gelatin scaffold on bone marrow stromal cells. 90,000 stromal cells were transferred to each of the 6 well plate sinks. In addition, in the test groups, scaffolding was added in amount of 6 mg, then the cells were cultured in the incubator for 72 hours under standard conditions of temperature and humidity. After that, 150 ml of the medium in both test and control groups was removed and 150 micro-liters of a solution of MTT (Sigma) was added and the cells were incubated for 2 hours. Finally, dimethyl sulfoxide (DMSO) (Sigma) was added and after 15 minutes shaking, a colored solution was obtained. The solution was measured by ELISA Reader at wavelengths of 530 and 630.
2.5. In vivo study design
In this experimental study, 15 adult male Wistar rats weighing 200-250 g were used. The animals were kept in the animal house of Mazandaran University of Medical Sciences at 22 ± 2° C and 12-hour alternating light. The study included three groups (n=5 in each group) covering two treatment groups and a control group. Using dentistry drills an injury with a diameter of 7 mm was made in the parietal bone close to the center line in each group. Group 1 (control group): Injury without transplantation (blank defect), group 2: implanted with hydroxyapatite-gelatin scaffold, group 3: hydroxyapatite-gelatin seeded with BMSCs.
2.5.1. Induction of critical-sized bone defect
Under sterile circumstances, rats in different groups were anesthetized by intraperitoneal injection of ketamine hydrochloride (40 mg/kg) and xylazine hydrochloride (10 mg/kg) (Merck-Germany). When the animals were completely anesthetized, the target area at the top of the skull was shaved by conventional blades. Using a sterile scalpel, an incision was made from between the two ears to the lower eye area. After that the skin and the periosteum were eliminated. Using a dentistry drill the target wounds which had a circular shape with a diameter of seven millimeters, were created in the parietal bones in the center line and at an equal distance from the temporalis muscle and the sagittal fissure. During the surgery, the lesions were washed several times with a sterile solution of PBS (0 Molar) and the bone above the dura matter was removed without damaging the middle meningeal artery. After recovery, the animals were transported to the animal house and kept under standard conditions of food, water and light.
2.6. In vivo characterizations
One week and one month after surgery, the animals were sacrificed and the wound region was removed with a bit of the host margin bone and each sample was placed in a small glass. In order to fixation, the samples were stored in a solution of 10% formalin for a whole week and decalcified by placing each sample in a solution of 14% EDTA (Gibco) for 16 days as a calcium challenger solution. Processing and preparation of blocks were performed according to standard methods of tissue preparation including dehydration, clearing and colonization. A block was created from each sample and every block was serially cleaved into 10 slices with 7 micrometers thickness and stained with hematoxylin-eosin for observing the presence of inflammatory cells and tissue repair. Trichrome staining (Trichrome Mason) was used toexamine the synthesis of collagen fibers (19). Newly bone formation in all experimental groups was scored as grade 0 to grade 4. The criteria for scoring is listed in Table 1.
Table 1. Semi-quantitative scale for estimation of bone formation.
| Score | Extent of bone in Transplant |
|---|---|
| 0 | No bone evident |
| 1 | Minimal bone evident (one trabecular per section) |
| 2 | Low bone formation, occupying only small portion of section |
| 3 | Moderate bone formation, occupying a substantial portion but less than one-half of the section |
| 4 | Abundant bone formation, occupying greater than one-half of the section |
Statistical analysis: The results were analyzed using SPSS version16 software (Chicago, USA), one-way analysis of variance (ANOVA) and paired t-test, and presented as mean ± SD. P <0.05 was considered significant.
3. RESULTS
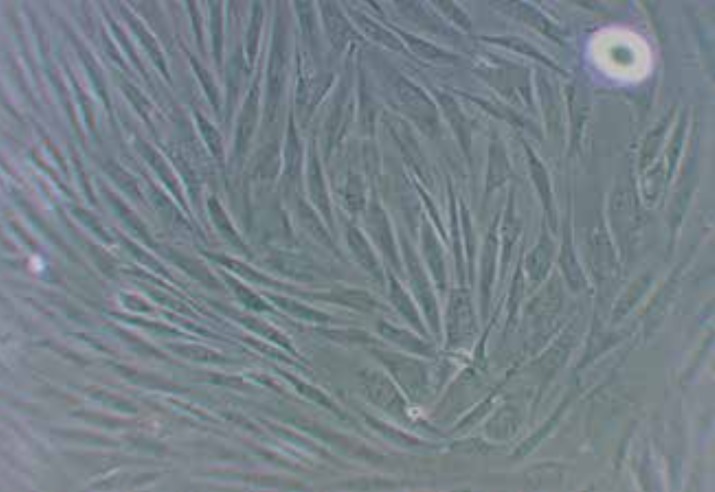
Bone marrow mesenchymal stem cell culture at early passages revealed a variety of cellular phenotypes including cells with an orbicular morphology and non-sticky and after successive passages their numbers were reduced significantly. In addition, bone marrow mesenchymal stem cells usually reach 70-80 percent density within 4 to 5 days. Microscopic examination of cells with the passage of 3 to 5 proved the appearance of spindle-shaped and fibroblast-like cells, indicating bone marrow mesenchymal stem cells (Figure 1).
Figure 1. Phase contrast microscopy image of mesenchymal stem cells cultured in the fourth passage. The existence of fibroblast-like cells and spindle shaped cells were observed. (400×).
3.1. In vitro characterizations
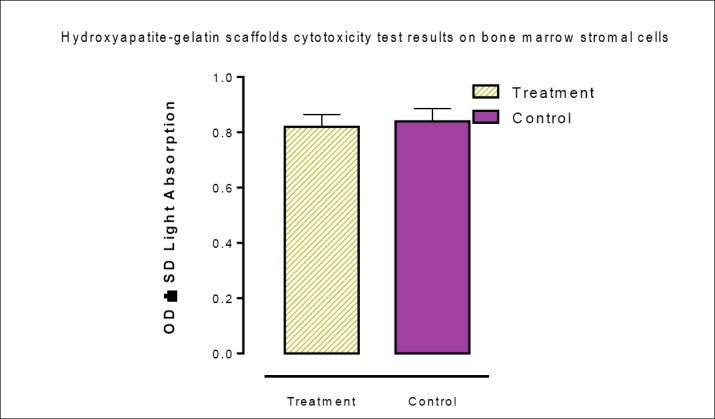
MTT results showed that the viability of the cells in the treatment and control groups were 0.85 ± 0.97 and 0.89 ± 0.02,respectively. Comparison of these two means showed that scaffolds did not induce toxic effects against bone marrow stromal cells (Figure 2).
Figure 2. Cytotoxicity effects of hydroxyapatite-gelatin scaffolds on bone marrow stromal cells (P<0.05).
3.2. In vivo characterizations
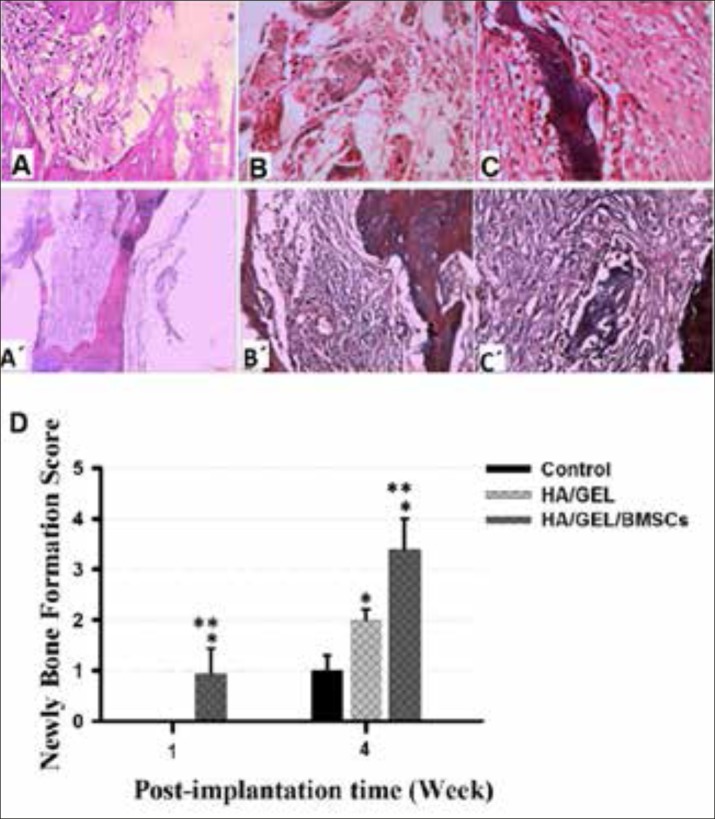
Histopathological findings showed the first signs of the onset of bone formation appeared in the first week in the transplanted Nano-hydroxyapatite - gelatin with mesenchymal stem cells group, as callus connective tissue was formed around the scaffold, and in some cases entered into the pores of the scaffolding. In addition,cancellous bone was formed in the middle of the cavity. In the fourth week, bone formation was much more completed and the remnants of the scaffold were found as islands inside the cancellous bone. Trichrome staining showed the high collagen deposition in animals received BMSCs-seeded HA/GELNano composite (Figure 3: images C and C’). But after one week of the Nano-hydroxyapatite-gelatin composite transplant group, a loose connective tissue with an average thickness surrounded the scaffold. In fact, in the fourth week, the connective tissue around the collagen scaffold started the synthesis of collagen, and bone formation was observed at the edge of the hole, close to the graft site. (Figure 3: images B and B’). In the control group, a thin connective tissue filled the injury site and there was no sign of bone formation. (Figure3: images A and A’). According to the newly bone formation scoring, animals received HA/GEL/BMSCs construct showed the highest score of bone healing between experimental groups at both 1 and 4 weeks post-surgery times. In addition, the bone formation score in HA/GEL experimental groups was significantly higher than those in control group at week 4.
Figure 3. Histologic sections 4 weeks after transplantation in rat skull. (A), (B) and (C) are images of the control group , the Nano-hydroxyapatite -gelatin composite transplant group and the transplanted Nano-hydroxyapatite-gelatin with mesenchymal stem cell group, respectively (stained by hematoxylin and eosin) (400×). (A’), (B’) and (C) are images of the control group, the Nano-hydroxyapatite-gelatin composite transplant group and the transplanted Nano-hydroxyapatite-gelatin with mesenchymal stem cell group, respectively (stained by the Trichrome) (400×). Bone formation at the site of injury was shown with white arrows. No trace of bone tissue formation was observed in the control group. Newly bone formation scoring showed the positive effectiveness of BMSCs-seeded HA/GEL Nano composite in critical-sized bone defect healing during both short-term (1 week) and long-term (4 weeks) post-implantation. * and ** indicate significant difference with control and HA/GEL groups, respectively (p<0.05).
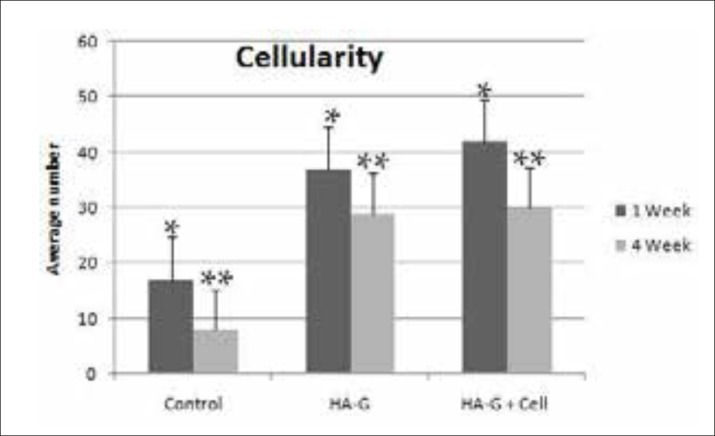
The presence of inflammatory cells (PMN’s) and all the recruited cells to the site of transplantation such as eosinophils (called cellularity orCell density), were investigated in different groups. The results showed that the presence of lymphocytes and polymorph nuclear in all of the groups. The highest number of lymphocytes in experimental groups were observed after one week of scaffold planting (Figure 4). Additionally, significant differences in the number of the polymorph nuclear cells available in the transplantation site were observed between the treatment and control groups and the maximum number of polymorph nuclear cells were found in the transplanted scaffold with mesenchymal cells after 4 weeks (Figure 4). The cellularity in the treated groups was much higher than the control group, both in the first and fourth weeks. However, no significant difference was observed between the two groups in which the scaffolding was used with and without stem cells (Figure 5).
Figure 4. The results of counting lymphocyte and polymorphonuclear cells at the transplantation union in the control, the Nano-hydroxyapatite -gelatin composite transplant and the transplanted Nano-hydroxyapatite-gelatin with mesenchymal stem cell groups. (A): A week after transplantation (B): Four weeks after transplantation (* P <0.05 & ** P <0.01).
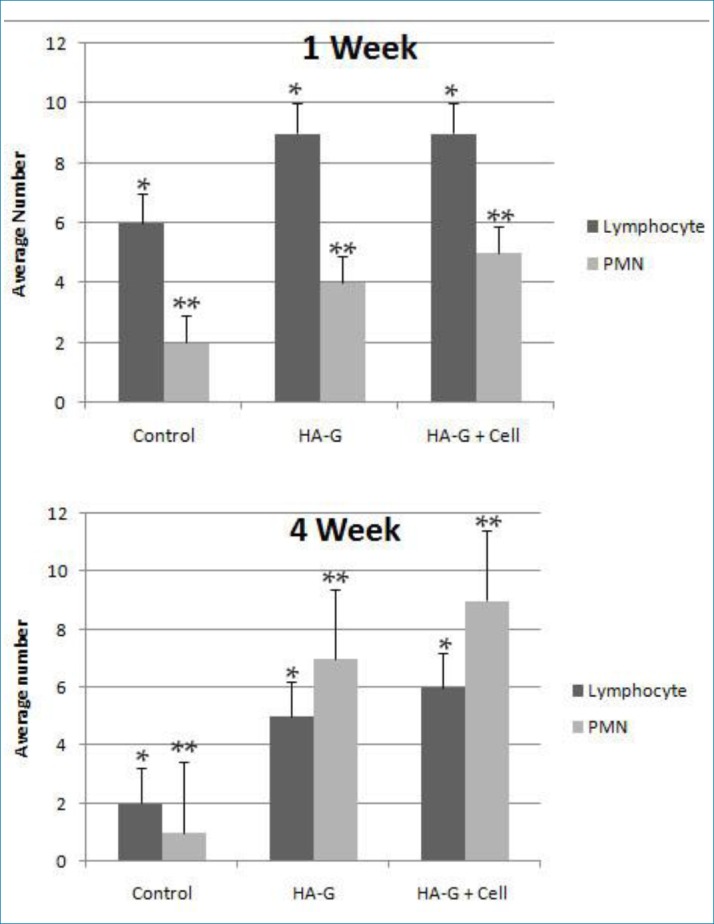
Figure 5. All the cells counted in the transplantation site (Cellularity) in the control, the Nano-hydroxyapatite -gelatin composite transplant and the transplanted Nano-hydroxyapatite-gelatin with mesenchymal stem cell groups (p<0.05).
4. DISCUSSION
Most bone injuries are healed spontaneously or with minimal clinical treatment. However, in some cases, due to various reasons, spontaneous healing doesn’t happen and further action is necessary. Currently, using tissue engineering techniques and scaffolding, healing large bone fractures can be achieved. Osteoblast cells cultured on scaffolds have been investigated in several studies and their potentialityin bone fracture healing have been demonstrated (18). Osteoblast cells synthesize and secrete a matrix that induce bone formation on the scaffold, facilitating the recovery. To obtain the best results from cell transplantation, cells should have features such as easy accessibility, rapid expansion in culture, immunology neutral, long term survival and compatibility with host tissue, suggestion BMSCs as a preferred choice (20).
In this study the stemness of bone marrow mesenchymal stem cells was explored. The fibroblast-like phenotype of bone marrow mesenchymal stem cells was retained during the successive passages. In addition, these cells possess features including colony formation, high proliferation and adhesion. The properties used in bone marrow mesenchymal stem cells in this research indicate the application of pure populations of these cells in terms of stemness, which are in line with the findings of previous researches (21). One of the characteristics of an ideal scaffold is providing space for cell proliferation. The results of cell cytotoxicity in our study showed a good biocompatibility of bone marrow mesenchymal stem cells on the scaffolds, consistent with previous studies (22).Bone marrow mesenchymal stem cells cultured on the scaffold are three-dimensional and similar to osteoblasts in bone matrix at in vivo conditions. There are several reports indicating the presence of signals in Extracellular matrix (ECM) which cause the expression of transcription factors and also affect some vital functions in the cell such as cell viability, migration, adhesion, growth, proliferation and cell differentiation (23). Histopathological evaluation on the rat skull revealed that hydroxyapatite-gelatin Nano composite transplantation with mesenchymal stem cells provides better results in bone defect repair as compared to other groups. As the first signs of the onset of bone formation showed itself as a connective callus around the scaffold in the first week , after four weeks bone formation was more complete and the thickness of the scaffolds were decreased and they were seen as islands. In addition, the dense collagen fibers in tissue samples were clearly visible. In this regard, hydroxyapatite-gelatin was recognized as a suitable scaffold for induction of bone formation (19). Moreover, the effects of bone matrix gelatin in the healing rat parietal bone defection was examined and it was showed that bone tissue regeneration with human bone matrix gelatin was much more faster (24). Study on hydroxyapatite-gelatin Nano composites with endometrial stem cells revealed significant formation of bone tissue (25). The use of hydroxyapatite-silk fibroin scaffold with bone marrow stromal cells completely repaired the forearm bone defect in rabbits (26). Many studies were conducted to design, synthesis and investigate the properties of Nano composites such as hydroxyapatite-collagen and hydroxyapatite-Silicon for bone healing and as an alternative to bones (27). A recent study showed that hydroxyapatite-silk fibroin scaffold, unlike the control group, did not change the biological nature of bone marrow mesenchymal stem cells. In addition, scanning electron microscopy micrographs showed that the cells migrated into the cavities and were connected to the scaffolds and their differentiation into osteoblasts was possible, suggesting the appropriate cell binding, high biocompatibility and lack of toxicity in hydroxyapatite-silk fibro in scaffolds (22). The main focus of the current research is a comparative study between inflammatory cells and cellularity cells in different groups. The amount of the inflammatory cells such as polymorphnuclear cells and lymphocytes and the amount of cellularity in the scaffold location zone and the host’s tissue were investigated. Polymorphnuclear cells and lymphocytes participate in response to absorption of scaffolding and in the immunological attack against the transplant, respectively. In previous study, we demonstrated that the hydroxyapatite-gelatin scaffolds show significant biocompatibility in an in vivo environment (28). The results showed that the highest number of lymphocytes in experimental groups were observed after one week of planting the scaffolds. The increase in the number of lymphocytes in experimental groups, compared to control groups, shows a rapid bone healing in the experimental groups. This is because lymphocytes act as feeding cells and have an important role in this process. In addition, reducing the number of lymphocytes, compared to polymorph nuclear cells, show a deceleration of the recovery from the first week to fourth week in the experimental groups, representing faster tissue repair in these groups, compared to the control group. However, during the four weeks after a bone injury, more presence of lymphocytes compared to the polymorph nuclear cells show the slower rate of feeding and restoration in the control group, compared to the experimental groups equipped with scaffolds. On the other hand, the migration of polymorphnuclear cells to the graft site showed a significant increase compared to the control group. This increase may be due to the phenomenon of degradation in which polymorphnuclear cells digest the scaffolds. In addition, increase of cellularity in the transplantation zone can provide useful information in the field of scaffolding analysis and progress in the reconstruction of bone tissue to the researcher, but further studies are necessary. Our study is in agreement with the results and Gholipour et al Azimi et al (22, 28). In a study at the first stage, fracture was covered with blood clots. After a few days, a loose connective tissue containing a large number of polymorphnuclear (due to acute inflammation) replaced the flocculation, and after a week the bone callus formed gradually and the dense connective tissue connected the two edges of the fracture together. After a month, the bone was formed and bone-forming cells and blood vessels were observed. In one-week samples, an increase in the number of polymorphnuclear and giant macrophage cells involved in scaffold digestion was observed. Similar to the results of the other studies, the cells were not significantly different in various groups. There were also no significant signs of chronic inflammation (lymphocytes, plasma cells and eosinophils) (23).
However, in this study, experimental groups in which hydroxyapatite scaffolds were used with and without stem cells derived from bone marrow, showed a similar cellularity in the first and fourth week. In the experimental groups carrying the scaffolds, a reduce in the number of lymphocytes from the first week to fourth week indicated the decreased need for feeding the growing bone-building cells and the evolving bone tissue by lymphocytes, meanwhile reduced the amount of chronic inflammation due to the presence of lymphocytes.
5. CONCLUSION
Since hydroxyapatite-gelatin is non-toxic and provides a perfect situation for growth, differentiation and cell migration, it seems that hydroxyapatite-gelatin scaffold reinforced with bone marrow mesenchymal stem cells is playing a pivotal role in bone healing and can be used as a useful therapeutic strategy for large bone defects.
Acknowledgment
This study was supported by the Immunogenic Research Center (IRC) of Sari Medical Faculty and Chancellor for Research and Technology of Mazandaran University of Medical Sciences (Project No: 93-499).
Authors’ contributions
Hatef Hasemi Hamidabadi, contribution to design ,acquisition, Majid Malekzadeh Shafaroudi, contribution to design, preparing and diagnosing the histologic samples; analysis and interpreting the histologic data, Morteza Seifi, contribution to analysis and interpretation of data,Maryam NazmBojnordi, contribution to analysis and interpretation of data,MasumeBehruzi, contribution to acquisition, analysis and interpretation of data, preparing the laboratory analysis of histologic samples, Mazaher Gholipourmalekabad, contribution toacquisition, analysis and interpretation of data;. Ali Malekzadeh Shafaroudi, translated the article, revising the article Nourollah Rezaei, contribution to acquisition, statistical analysis, drafting the article, provided endnote, final modification and submitted the article. All authors approved of the final manuscript.
Conflict of interest
no conflict of interest to declare.
REFERENCES
- 1.Wegman FPM, van der Helm YJ, Oner FC, Dhert WJ, Alblas J. Combination of bone morphogenetic protein-2 plasmid DNA with chemokine CXCL12 creates an additive effect on bone formation onset and volume. European cells & materials. 2015;30:1–11. doi: 10.22203/ecm.v030a01. [DOI] [PubMed] [Google Scholar]
- 2.Mobini SSHM, Hesarakl S, Gelinsky M. Fabrication and Characterization of Regenerated Silk/bioglass Composites for Bone Tissue Engineering. Modares Journal of Medical Sciences: Pathobiology. 2012;15(2):47–60. [Google Scholar]
- 3.Schmidmaier GCR, Wildemann B, Beque T, Lowenberg D. Bone morphogenetic proteins in critical-size bone defects: what are the options? Injury. 2009;40:s39–s43. doi: 10.1016/S0020-1383(09)70010-5. [DOI] [PubMed] [Google Scholar]
- 4.Porter JRRT, Popat KC. Bone tissue engineering: a review in bone biomimetics and drug delivery strategies. Biotechnology Progress. 2009;25(6):1536–60. doi: 10.1002/btpr.246. [DOI] [PubMed] [Google Scholar]
- 5.Salgado AJCO, Reis R L. Bone tissue engineering: state of the art and future trends. Macromolecular bioscience. 2004;4(8):743–65. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 6.Damien CJPJ. Bone graft and bone graft substitutes: a review of current technology and applications. Journal of Applied Biomaterials. 1991;2(3):187–208. doi: 10.1002/jab.770020307. [DOI] [PubMed] [Google Scholar]
- 7.Hollinger JOET, Doll B, Sfeir C. 1st. Boca Raton: CRC Press; 2004. Oct 14, Bone tissue engineering; pp. 20–80. 2004. [Google Scholar]
- 8.Runyan CMTJ. Clinical applications of stem cells in craniofacial surgery. Facial plastic surgery. 2010;26(5):385–95. doi: 10.1055/s-0030-1265017. [DOI] [PubMed] [Google Scholar]
- 9.Yunoki SIT, Monkawa A, Ohta K, Kikuchi M, Sotome S, Tanaka J. Control of pore structure and mechanical property in hydroxyapatite/collagen composite using unidirectional ice growth. Materials letters. 2006;60(8):999–1002. [Google Scholar]
- 10.Mozafar M, Gholipourmalekabadi M, Chauhan NPS, Jalali N, Asgari S, Caicedoa JC, Urbanska AM. Synthesis and characterization of nanocrystalline forsterite coated poly (l-lactide-co-β-malic acid) scaffolds for bone tissue engineering applications. Materials Science and Engineering. 2015;C(50):117–23. doi: 10.1016/j.msec.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Liao CF, Zhu Y. Osteoblasts adherence and migration through three-dimensional porous mineralized collagen based composite: nHAC/PLA. Journal of Bioactive & Compatible polymers. 2004;19(2):117–30. [Google Scholar]
- 12.Zhang SM CF, Liao SS, Zhu Y, Han L. Synthesis and biocompatibility of porous nano-hydroxyapatite/collagen/alginate composite. Journal of Materials Science: Materials in Medicine. 2003;4(7):641–5. doi: 10.1023/a:1024083309982. [DOI] [PubMed] [Google Scholar]
- 13.Dib M KM, Fathi M H, Gholipourmalekabadi M, Samadikuchaksaraei A. Preparation and characterization of polycaprolactone/forsterite nanocomposite porous scaffolds designed for bone tissue regeneration. Composites Science and Technology. 2012;72(6):716–23. [Google Scholar]
- 14.Kim H W, Salih V. Stimulation of osteoblast responses to biomimetic nanocomposites of gelatin–hydroxyapatite for tissue engineering scaffolds. Biomaterials. 225;26(25):5221–30. doi: 10.1016/j.biomaterials.2005.01.047. KHE. [DOI] [PubMed] [Google Scholar]
- 15.Wang JGMJ. Temporal and Spatial Independence of Bone and Cartilage Induction by Demineralized Bone Powder in Cranial Defects and Subcutaneous Tissues of Rats. Connective Tissue Research. 1996;34:116–70. [Google Scholar]
- 16.Ai JHKS, Azami M, Bahrami N, Mohamadnia A. Repair of critical size rat calvarial defects using endometrial-derived stem cells embedded within gelatin/apatite nanocomposite scaffold. Stem Cell Discovery. 2013;3:37–43. AIA. [Google Scholar]
- 17.Joe AW GEK. Mesenchymal stem cells and potential applications in treating ocular disease. Current eye research. 2010;35(11):941–52. doi: 10.3109/02713683.2010.516466. [DOI] [PubMed] [Google Scholar]
- 18.Moghadam HG SGK, Holmes HH, Clokie CM. Histomorphometric evaluation of bone regeneration using allogeneic and alloplastic bone substitutes. Journal of oral and maxillofacial surgery. 2004;62(2):202–13. doi: 10.1016/j.joms.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Bancroft JD SA. Churchill Livingstone Publication Company; 1990. Theory and Pracice Histological Techniques. [Google Scholar]
- 20.Kale AA DCPE. Osteoinductive agents. Basic science and clinical applications. American journal of orthopedics (Belle Mead, NJ) 1995;24(10):752–61. [PubMed] [Google Scholar]
- 21.Baghbani FMF, Nazari AG, Kamran AR, Tondnevis F, Nezafati N, Mozafari M. Biological response of biphasic hydroxyapatite/tricalcium phosphate scaffolds intended for low load-bearing orthopaedic applications. Advanced Composites Letters. 2012;12(1):16–24. [Google Scholar]
- 22.Gholipourmalekabadi MMM, Gholipourmalekabadi M, Nazm Bojnordi M, Hashemi-soteh MB, Salimi M, Rezaei N, Ghasemi Hamidabadi H. In vitro and in vivo evaluations of three-dimensional hydroxyapatite/silk fibroin nanocomposite scaffolds. Biotechnology and applied biochemistry. 2015;62(4):441–50. doi: 10.1002/bab.1285. [DOI] [PubMed] [Google Scholar]
- 23.Kagami HAH, Tojo A. Bone marrow stromal cells (bone marrow-derived multipotent mesenchymal stromal cells) for bone tissue engineering: basic science to clinical translation. The international journal of biochemistry and cell biology. 2011;43(3):286–9. doi: 10.1016/j.biocel.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Takaoka KNH, Yoshikawa H, Masuhara K, Tsuda T, Ono K. Ectopic bone induction on and in porous hydroxyapatite combined with collagen and bone morphogenetic protein. Clinical orthopaedics and related research. 1988;234:250–4. [PubMed] [Google Scholar]
- 25.Scott CK HJA. The matrix of endochondral bone differs from the matrix of intramembranous bone. Calcified tissue international. 1991;49(5):349–54. doi: 10.1007/BF02556258. [DOI] [PubMed] [Google Scholar]
- 26.Yamashita K TT. Ultrastructural observation of calcification preceding new bone formation induced by demineralized bone matrix gelatin. Cells Tissues Organs. 1992;143(4):261–7. doi: 10.1159/000147260. [DOI] [PubMed] [Google Scholar]
- 27.Aminian A SHM, Samadikuchaksaraei A, Bakhshi F, Gorjipour F, Farzadi A, Schmücker M. Synthesis of silicon-substituted hydroxyapatite by a hydrothermal method with two different phosphorous sources. Ceramics International. 2011;37(4):1219–29. [Google Scholar]
- 28.Azami MTS, Samadikuchaksaraei A, Hashjin M S, Baheiraei N, Kamali M, Nourani MR. A porous hydroxyapatite/gelatin nanocomposite scaffold for bone tissue repair: in vitro and in vivo evaluation. Journal of Biomaterials Science, Polymer Edition. 2012;23(18):2353–68. doi: 10.1163/156856211X617713. [DOI] [PubMed] [Google Scholar]


