Figure 2. Gene delivery vectors used for gene therapy of PID.
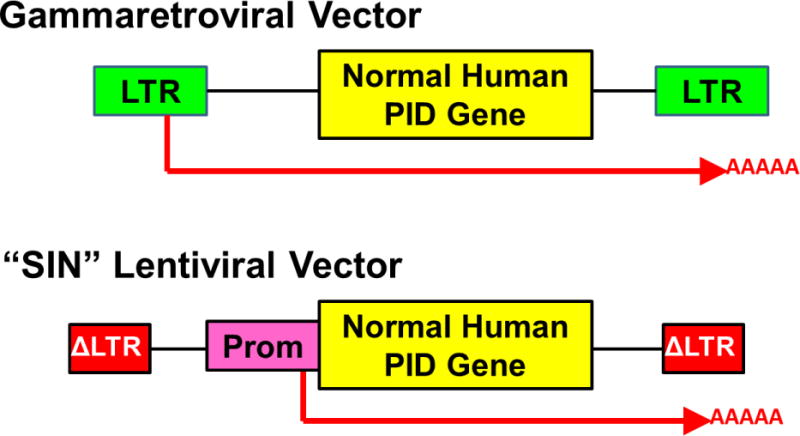
Upper: Gammaretroviral vectors have viral long terminal repeats (LTR) at each end with viral enhancers and promoters that drive transcription and termination/polyadenylation of a normal copy of the relevant human gene involved in the inherited PID. The messenger RNA produced from the vector is shown as a red arrow including the polyA tail. Lower: Self-inactivating (“SIN”) lentiviral vector has LTR with the enhancers deleted (Δ) and an internal promoter (Prom) to drive transcription of the human PID gene. In current clinical trials, lentiviral vectors being used for ADA SCID and XSCID are using the human Elongation Factor Alpha-1 gene short promoter (EFS) to drive the ADA and IL2Rg genes, respectively, the WASP gene endogenous promoter to drive a WASP gene, and a chimeric myeloid promoter to drive the gp91phox gene for XCGD.
